Experimental Part
The adsorption of phenol and pyridine vapors was studied in statistical vacuum conditions. An adsorption sleeve with spring tungsten scales served as the reactor. Spiral sensitivity: 4,2∙10-4 g/mm. Measurements were performed using cathetometer KM-6. The pyridine pressure was measured with a help of U - shaped manometer, at the phenol adsorption was used McLeod gauge. After the adsorption, desorption of vapor adsorbates was carried out by means of gradual decompression by the removal of a part of vapor in forevacuum system.
Outcomes Discussion
The method of adsorption of molecules, mainly organic acids and bases, is used for the characteristic of the acid-base sites of solid surface.
For pyridine and phenol adsorption from a vapor phase the full sorption-desorption isotherms of phenol and pyridine on the investigated oxides are received (fig. 1, 2). At pyridine adsorption, the isotherm has the S-shaped form; at phenol adsorption, the isotherm form is typical for monomolecular adsorption with a convex initial section. At small relative pressure the dependence of adsorption rate on the value of a sample´s specific surface is visible: it is possible to rank them upon the value of Sspecific: TiO2 (anatase) > ZrO2 > HfO2 > TiO2 (rutile) - 159;45,7;35; 11,4 m2/g respectively [1]. And the time of adsorption equilibrium of phenol and pyridine is 3 hours, 1 hour and 30-45 minutes. It allows us to say that at small relative pressure sorption is mainly defined by the surface factor, and structural features begin to appear only after the formation of several molecular layers [2].
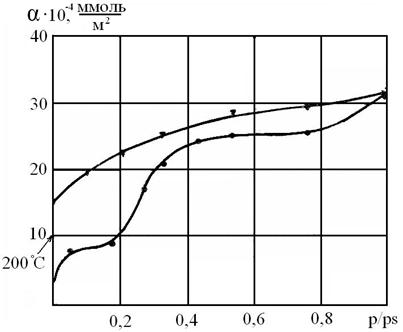
Figure 1. ● Sorption - ▼ - desorption isotherm of pyridine on ZrO2 at 293 ºК
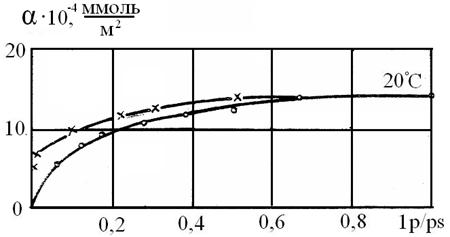
Figure 2. ● Sorption - х - desorption isotherm of phenol on HfO2 at 293 ºК
At bigger relative pressure (p/ps = 0,7÷1) the multimolecular pyridine adsorption takes place. The delay in desorption is observed, and a hysteresis loop is formed, and the hysteresis continues throughout the range of relative pressures. All experimental isotherms of pyridine, presented in logarithmic coordinates of the Brunauer-Emmet-Teller equation, look like straight lines up to p/ps = 0,3, this fact enabled us to calculate the amount of adsorbed substance at a monolayer covering.
For the detailed investigation of interaction in the mentioned gas-adsorbent systems the heats of adsorption were calculated using the Clapeyron-Clausius equation. The initial heats of pyridine adsorption are (20-34) kJ / mol, that can be characteristic for a case of hydrogen bonding. The latter may result from the interaction of proton hydrogen of free hydroxyl groups on the surface with free electron pairs of nitrogen atom of pyridine functional group [3]. The calculation of vapor-phase phenol showed the high initial heats of adsorption of 71-83 kJ / mol (Fig. 3). This fact indicates the existence of the adsorption sites with high activity on the surface of the investigated oxides. According to [3], the adsorption of phenol molecules with lone-electron pair can proceed to donor-acceptor mechanism which is especially probable in the oxides of titanium subgroup elements with unfilled d - orbitals of the atoms Ti, Zr, Hf (IInd type sites). In this case, the energy and the character of donor-acceptor interaction, i.e. the degree of electron transition from the molecule to the metal atom, strongly depend on the interaction partners and may vary largely. The proportion values of the heats of pyridine adsorption from n-hexane and from the gas phase indicate a lack of coordination bonds of that adsorbate, but they do not deny the presence of Lewis sites in any case [4].
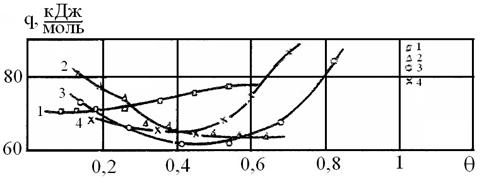
Figure 3. Differential heats of phenol adsorption from a vapor phase:
1 on rutile, 2 on anatase, 3 - on zirconium oxide, 4 - on hafnium oxide
The molecule platform in the adsorbed layer indicates the different interaction of the adsorbed molecules with a surface (table 1). At adsorption from the vapor phase, the adsorbate molecules are exposed to such factors as the adsorbate area, and the area of vapor phase, which, actually, can be neglected. Ultimately, it is energetically profitable for adsorbate to adsorb "flat".
In order to clarify the bonds´ character of the adsorbed pyridine and phenol molecules with an adsorbents surface, it is interesting to look at the results of investigation of adsorption from a vapor phase. Under pumping-out at temperature 293 K and pressure 133,3∙10-5 Pa (53-36,5 %) of the adsorbed pyridine and (50-22,5%) - phenol remain on the oxides surface.
With increasing the pumping-out temperature up to 473 К (38,6-21,7 %) of pyridine and (41-11,2 %) of phenol remain. According to the amount of irreversibly linked pyridine and phenol at 473K, the number of acid and basic sites is calculated. The TiO2 (rutile) possesses the greatest number of the acid sites: 20∙1017 per m2, and number of the basic sites equals 3,7∙1017 per m2. The anatase surface contains slightly more basic sites, than the acid ones (14,69∙1017 per m2 and 12,3∙1017 per m2 respectively). The number of the acid sites on ZrO2 and HfO2 is (5-9∙1017) per m2, and the number of the basic sites is (3,1 - 3,6∙1017) per m2.
According to the force of the acid sites, it is possible to rank the investigated samples: TiO2 (rutile) > ZrO2 > HfO2 > TiO2 (anatase). This is indicated by the data on hydrogen index of an isoelectric state of oxides [1], the phenol and pyridine adsorption from n-hexane [4] and the data on bond strength of phenol and pyridine with the surface oxide from the gas phase.
Table 1
Data of pyridine and phenol adsorption from a vapor phase
on oxides of elements from titanium sub-group
|
oxides |
Pyridine |
||||||||||||
|
273 ºК |
293 ºК |
Number of chemosorption sites of adsorbates х 1017 per m2 (desorption at 473 ºК) |
|||||||||||
|
Monolayer capacity αm∙10-3 mmol/m2
|
ω,
|
Quantity of irreversibly connected adsorbates at 273 ºК |
Quantity of irreversibly connected adsorbates at 473 ºК |
Monolayer capacity αm∙10-3 mmol/m2 |
ω,
|
Quantity of irreversibly connected adsorbates at 293 ºК |
Quantity of irreversibly connected adsorbates at 473 ºК |
||||||
|
α ∙10-3 mmol/m2 |
% |
α∙10-3 mmol/m2 |
% |
α∙10-3 mmol/m2 |
% |
α∙10-3 mmol/m2 |
% |
||||||
|
TiO2 (r) |
2,4 |
68,8 |
2,59 |
57,6 |
1,43 |
31,8 |
5,6 |
53,6 |
4,59 |
53,4 |
3,33 |
38,7 |
20,04 |
|
ZrO2 |
0,8 |
57,3 |
0,63 |
42,6 |
0,37 |
24,7 |
0,8 |
50,7 |
1,59 |
48,5 |
0,9 |
27,6 |
5,43 |
|
GfO2 |
2 |
85,3 |
3,73 |
61,5 |
1,37 |
22,6 |
2,4 |
69,6 |
2,24 |
36,5 |
1,6 |
26,1 |
9,64 |
|
TiO2 (an) |
3,8 |
43,4 |
4,36 |
54,3 |
1,39 |
17,3 |
8,2 |
25,4 |
3,6 |
38,4 |
2,04 |
21,8 |
12,3 |
|
phenol |
|||||||||||||
|
TiO2 (r) |
11,99 |
13,8 |
6,04 |
50,04 |
1,64 |
13,7 |
5,5 |
30,0 |
1,25 |
22,5 |
0,62 |
11,2 |
3,73 |
|
ZrO2 |
85,8 |
2 |
80,5 |
93,7 |
33,2 |
38,6 |
1,83 |
90,8 |
0,71 |
38,8 |
0,5 |
28,9 |
3,2 |
|
GfO2 |
34,14 |
4,9 |
33,8 |
98,9 |
16,4 |
47,9 |
1,44 |
115,7 |
0,72 |
50,2 |
0,6 |
41,8 |
3,6 |
|
TiO2 (an) |
9,7 |
21,2 |
8,56 |
88,2 |
2,91 |
30,1 |
7,8 |
17,1 |
2,8 |
36 |
2,4 |
31,1 |
14,7 |
References
- Skripko T.V. The adsorption properties of titanium subgroup oxides / / Modern high technologies. - 2007. - № 9 - p. 41-42.
- Kirovskaya I.A. Surface Phenomena: monography. - Omsk: Omsk State Technical University Publishing House, 2001, - 175 p.
- Kiselev V.F. / / Kinetics and Catalysis. - 1970. - Vol.11 - № 2. - p. 403.
- Skripko T.V. Adsorption studies of acid-base surface properties of titanium subgroup oxides / / Modern high technologies. - 2008. - № 9. p. 55 - 56.

