Introduction
Acute viral hepatitis continues to be one of the urgent global health problems, especially in endemic regions with limited access to safe drinking water and adequate sanitation. Among enteral viral infections, hepatitis A virus (HAV) and hepatitis E virus (HEV) are the main causative agents of acute liver damage, having similar clinical manifestations, but differing in epidemiology and pathogenesis [1, 2].
Hepatitis E virus infection, previously considered to be characteristic only of endemic territories, is now recognized as widespread worldwide and capable of causing severe clinical forms, especially among pregnant women and people with immunodeficiency [3]. The hepatitis A virus, on the contrary, is traditionally associated with a benign course of the disease in children and young adults in regions with unsatisfactory sanitary conditions [4, 5, 6].
Currently, HEV is divided into eight genotypes. Genotypes 1 and 2 infect exclusively humans, while genotypes 3, 4, and 7 can infect both humans and animals. And genotypes 5, 6, and 8 are limited to infecting animals. People with weakened immune systems may also develop chronic hepatitis and extrahepatic manifestations, including neurological disorders [7].
The results of epidemiological surveillance in the southern regions of the Kyrgyz Republic revealed changes in the age structure of morbidity and severity of acute viral hepatitis E and A, which requires a detailed comparative analysis of their clinical and laboratory characteristics. This is important for improving diagnostic accuracy and optimizing patient management tactics [8].
The present study is aimed at a comparative assessment of the clinical manifestations and laboratory parameters of acute viral hepatitis E and acute viral hepatitis A among adult patients hospitalized in infectious diseases hospitals in the southern region of Kyrgyzstan. Identifying key differences and similarities between the two forms of infection will improve diagnosis, prognosis, and treatment tactics in endemic areas.
The aim of the study is to present the clinical and laboratory manifestations of acute viral hepatitis E in a comparative aspect with viral hepatitis A among adult patients.
Materials and methods of research
In this retrospective study, we analyzed data from adult patients with acute viral hepatitis E (AVHE) (n=134) and acute viral hepatitis A (AVHA) (n=126) who were hospitalized in the infectious diseases departments of the Osh Regional United Clinical Hospital, Jalal-Abad Regional Clinical Hospital, and Batken Regional Clinical Hospital in the southern regions of the Kyrgyz Republic.
The study period covered the years 2006 to 2025 as part of a sentinel epidemiological surveillance program for acute viral hepatitis, conducted in collaboration with the US Centers for Disease Control and Prevention (CDC), during the seasonal increase in acute viral hepatitis cases in the endemic areas of the country.
The study also used materials from serological studies that were conducted as part of a research project titled “Creating test systems for the serological diagnosis of hepatitis E and testing their diagnostic effectiveness on clinical material from endemic and non-endemic regions”. The project was conducted in Russia, Belarus and the Kyrgyz Republic between 2015 and 2020.
The etiological diagnosis of acute respiratory virus infection was made based on the serological detection of antibodies to HEV IgM and HEV IgG using test systems produced by Xantai-Hepe (China) and local manufacturers by ELISA. To confirm the diagnosis in patients with positive results for anti-HEV IgM, HEV RNA was also determined using primers developed by the CDC. HAV diagnosis was established based on detection of anti-HAV IgM antibodies using Vector-Best test systems (Russia). Patients with markers for hepatitis A, B, C or D were excluded from the AVHE group.
The clinical characteristics included an analysis of medical documentation with the identification of demographic data, symptoms of the disease and features of the course in the pre-jaundice and jaundice periods. The severity of the disease was classified into mild, moderate and severe forms.
Laboratory tests also determined the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total and direct bilirubin, thymol test results, prothrombin index, total protein, albumin, cholesterol levels and alkaline phosphatase activity.
Statistical analysis was performed using Microsoft Excel 2021 (Microsoft Corporation, USA) and Epi Info 6 (CDC, USA). The data were presented as averages and standard errors of the mean (M±m) with 95% confidence intervals (CI) calculated. The Student’s criterion was used to compare the groups; the differences were considered statistically significant at p<0.05. Correlation analysis was performed using the Pearson method.
The ethical approval of the study was obtained in accordance with national requirements for conducting research with human participation.
Results of the research and discussions
People aged 18 to 19 years (52.4%) were highly susceptible to AVHA disease (Figure 1). It is important to note that in most cases, patients in the age group of 30-39 years were ill with AVHE. And these data confirm that the main role in maintaining the epidemiological potential of this form of viral hepatitis belongs to patients of this group of patients. It should also be noted that cases of AVHE, unlike AVHA, have also been reported in patients over the age of 50. The sexual structure of AVHE was dominated by male patients, accounting for 65,6%, and among women it was observed in 34,4% of cases. We did not observe any special differences in the proportion of men and women with AVHA.
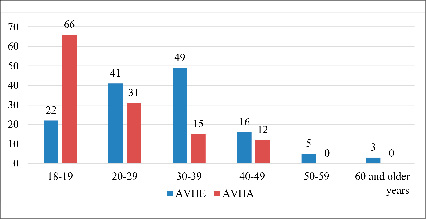
Fig. 1. Age structure of patients with AVHE and AVHA (abs.)
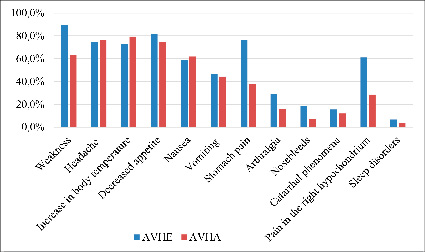
Fig. 2. Comparative characteristics of clinical symptoms in the pre-jaundice period in AVHE and AVHA
In acute viral hepatitis E, the moderate form was diagnosed in 106 patients (79,0%), the severe form in 28 (21,0%) patients, and the mild forms in the observed patients were not registered. In the group of patients with acute viral hepatitis A, the moderate form was diagnosed in 90 patients (71,5%), the severe form in 17 (13,5%) and the mild forms were diagnosed in 19 patients (15,0%).
The duration of the pre-jaundice period in acute viral hepatitis E was 7,9±0.3 days, and in acute viral hepatitis A – 5,3±0,2 days (7,9±0,2; 5,3±0,2; P>0,001). In the group of patients with acute viral hepatitis E, acute onset of the disease was registered in 76,0% of patients, and in 24,0% – subacute onset, and in the group of patients with acute viral hepatitis A, acute onset of the disease was registered in 87,0% of patients, and in the remaining patients, 13,0% – subacute onset. In both acute and subacute cases of both forms of viral hepatitis, clinical signs of viral hepatitis were already detected in the pre-jaundice period.
It follows from the data in Figure 2 that the main symptoms of AVHE in the initial or pre-jaundice period were the same symptoms that were observed in patients with AVHA. However, the presented comparative assessment of the frequency of the main clinical symptoms in the pre-jaundice period in patients with AVHE and AVHA showed that patients with AVHE were significantly more likely to experience symptoms such as weakness, stomach pain and right hypochondrium pain, arthralgia, and nosebleeds compared with patients with AVHA. At the same time, there was no significant difference in other symptoms between AVHE and AVHA in the pre-jaundice period. At the end of the pre-jaundice period, acholia and discoloration of urine were noted in all patients.
Table 1 shows data on a comparative assessment of the duration of pre-jaundice clinical manifestations in AVHE and AVHA. The table data in the pre-jaundice period indicate a significant increase in the duration of symptoms of weakness (p<0,01) and stomach pain (p<0,05) in patients with AVHE compared with AVHA, which indicates a more pronounced prodromal course of hepatitis E. At the same time, there were no significant differences in the duration of other clinical symptoms in AVHE and AVHA. The duration of the pre-jaundice period in acute viral hepatitis E was significantly longer and amounted to 7,9±0,3 days compared with acute viral hepatitis A – 5,3±0,2 days (P<0,001).
With the onset of the jaundice period, there was a significant increase in dyspeptic phenomena and intoxication symptoms in the group of patients with acute viral hepatitis E and in the group of patients with acute viral hepatitis A, the intensity of symptoms decreased (Fig. 3).
Table 1
Duration of clinical symptoms in the pre-jaundice period with AVHE and AVHA (in days)
|
Clinical symptoms |
AVHE (n=134) |
AVHA (n=126) |
Р |
||
|
М±m |
95% CI |
М±m |
95% CI |
||
|
Increase in body temperature |
2,8±1,2 |
0,4-5,2 |
2,5±1,1 |
2,3-4,7 |
Р>0,05 |
|
Headache |
3,5±1,3 |
0,9-6,1 |
2,8±0,9 |
1,0-4,6 |
P >0,05 |
|
Weakness |
6,4±1,4 |
3,6-9,2 |
2,3±0,8 |
0,7-3,9 |
P <0,01 |
|
Decreased appetite |
4,6±1,5 |
1,6-7,6 |
3,4±0,8 |
1,8-5,0 |
P >0,05 |
|
Nausea |
3,5±1,3 |
0,9-6,1 |
3,2±1,1 |
1,0-5,4 |
P >0,05 |
|
Vomiting |
2,5±0,7 |
0,9-3,9 |
2,1±0,8 |
0,5-3,7 |
P >0,05 |
|
Stomach pain |
6,1±1,2 |
3,7-8,5 |
2,0±0,7 |
0,6-3,4 |
P <0,05 |
|
Catarrhal phenomenon |
2,8±1,2 |
0,4-5,2 |
3,7±1,4 |
0,9-6,5 |
P >0,05 |
|
Arthralgia |
4,5±1,8 |
0,9-8,1 |
2,5±0,6 |
1,3-3,7 |
P >0,05 |
|
Nosebleeds |
2,5±0,9 |
0,7-4,3 |
1,3±0,5 |
0,3-2,3 |
P >0,05 |
|
Pain in the right hypochondrium |
5,2±1,0 |
3,2-7,2 |
3,5±0,2 |
3,1-3,9 |
P >0,05 |
|
Sleep disorders |
1,2± 0,3 |
0,6-1,8 |
1,3±0,2 |
0,9-1,7 |
P >0,05 |
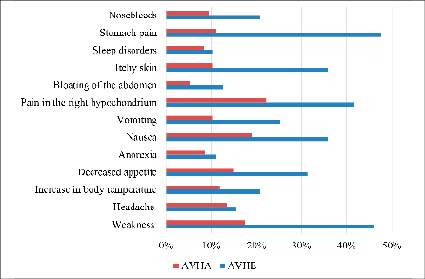
Fig.3. Comparative characteristics of clinical symptoms in the jaundice period in AVHE and AVHA
It was found that headache, anorexia and sleep disorders were observed with the same frequency in both groups (p>0,05). However, symptoms such as itching of the skin and dyspeptic manifestations were significantly more common in patients with AVHE (p<0,05). It should also be noted that patients with AVHE in the jaundice period were significantly more likely to have the following symptoms: weakness, pain in the right hypochondrium, nosebleeds and bloating compared with the AVHA group. These data highlight the features of the course of the jaundice period in various etiological forms of enteric viral hepatitis.
Table 2
Duration of symptoms of the jaundice period in AVHE and AVHA (in days)
|
Clinical symptoms |
AVHE (n=134) |
AVHA (n=126) |
Р |
||
|
М±m |
95% CI |
М±m |
95% CI |
||
|
Increase in body temperature |
3,8±1,5 |
0,8-6,8 |
2,5±1,2 |
0,1-4,9 |
Р > 0,05 |
|
Headache |
4,1±1,7 |
0,7-7,5 |
3,2±1,1 |
1,0-5,4 |
P >0,05 |
|
Weakness |
8,1±1,5 |
5,1-11,1 |
2,1±0,3 |
1,5-2,7 |
Р < 0,001 |
|
Anorexia |
2,4±0,2 |
2,0-2,8 |
1,7±0,1 |
1,5-1,9 |
Р < 0,05 |
|
Decreased appetite |
4,7±1,3 |
2,1-7,3 |
1,8±0,2 |
1,4-2,2 |
Р > 0,05 |
|
Nausea |
5,1± 0,8 |
3,5-6,7 |
2,0±0,5 |
1,0-3,0 |
Р < 0,05 |
|
Vomiting |
5,3±0,5 |
4,3-7,3 |
2,1±0,2 |
1,7-2,5 |
Р < 0,05 |
|
Stomach pain |
9,3±2,3 |
4,7-13,9 |
3,5±1,0 |
1,5-5,5 |
Р < 0,05 |
|
Bloating of the abdomen |
4,6±0,8 |
3,0-6,2 |
2,4±0,3 |
1,8-3,0 |
Р < 0,05 |
|
Itchy skin |
7,2± 1,6 |
4,0-10,4 |
3,1±1,2 |
0,7-5,5 |
Р < 0,05 |
|
Sleep disorders |
3,0±0,5 |
2,0-4,0 |
2,1±0,4 |
1,3-2,9 |
Р > 0,05 |
|
Pain in the right hypochondrium |
7,3±2,1 |
3,1-11,5 |
3,1±0,4 |
2,3-3,9 |
Р < 0,05 |
|
Joint pain |
4,3±1,2 |
1,9-6,7 |
2,0± 0,1 |
1,8-2,2 |
Р < 0,05 |
A comparative characteristic of the duration of the clinical manifestations of the jaundice period in AVHE and AVHA is shown in table 2. The data obtained indicate that there are some significant differences in the duration of clinical symptoms during this period. Statistically significant prolongation of symptoms, including weakness (p<0,001), anorexia (p<0,05), nausea (p<0,05), vomiting (p<0,05), stomach pain (p<0,05), pruritus (p<0,05) and pain in the right hypochondrium (p<0,05), was noted in patients with AVHE, which indicates a more severe clinical course of the jaundice period of this variant of hepatitis.
The severity of jaundice in patients with AVHE was mild in 15 (11,2%), moderate in 91 (67,8%) and significantly more pronounced in 28 (21,0%) compared with AVHA (15,2±3,8 and 7,4±1,3; P < 0,05). In contrast to AVHA, 11,0% of patients with AVHE had a cholestatic form of the disease with a prolonged progressive course with a further transition to a chronic form. This form of the disease was mainly found among 68,5% of male patients.
Liver enlargement in acute viral hepatitis non-A -non -E was registered in 91%, and in acute viral hepatitis A – 89,6%. The liver was painful on palpation in both cases of hepatitis. It should be noted that splenomegaly was twice as common in patients with AVHE (20,8%) as in patients with AVHA (9,5%).
Table 3 presents data from laboratory monitoring of patients with AVHE and AVHA. In AVHE, higher activity values of ALT (p<0,05), total bilirubin (p<0,001) and direct bilirubin (p<0,01), thymol test (p<0,05), and alkaline phosphatase (p<0,01) were noted. At the same time, the albumin level is statistically significantly lower in AVHE (p<0,05), which may indicate a more pronounced violation of synthetic liver function. The differences in other biochemical parameters were statistically insignificant (p>0,05).
It is interesting to note that 4 pregnant women were among those with AVHE. However, the disease was moderate in all cases and there were no complications. After further follow-up, pregnancies and childbirth proceeded smoothly as well. The average length of stay in the hospital for acute viral E was 16,3±7,4 days, while for acute viral hepatitis A it was 11,6±4,7 days (16,3±7,4; 14,6±6,94; P>0,05).
The study also analyzed correlations between the level of total bilirubin and biochemical markers (alkaline phosphatase, albumin) in patients with severe acute viral hepatitis E and acute viral hepatitis A.
Figure 4 shows a strong positive correlation between the level of total bilirubin and the activity of alkaline phosphatase in patients with severe AVHE (correlation coefficient r = 0,81; n = 28). This indicates a pronounced relationship between an increase in cholestatic syndrome and an increase in bilirubinemia in this type of viral liver damage.
Table 3
Comparative characteristics of the results of biochemical blood tests in patients with AVHE and AVHA
|
Laboratory parameters |
AVHE (п=134) |
AVHA (п=126) |
Р |
||
|
M±m |
95% CI |
M±m |
95% CI |
||
|
ALT (un/l) |
12,3±0,2 |
11,9-12,7 |
11,2±0,3 |
10,6-11,8 |
Р < 0,05 |
|
AST (un/l) |
10,8±0,2 |
10,4-11,2 |
10,3±0,2 |
9,9-10,7 |
Р > 0,05 |
|
Total bilirubin, (mcmol/l) |
145,0±6,3 |
132,4-157,6 |
98,0±5,2 |
87,6-108,4 |
Р < 0,001 |
|
Direct bilirubin (mcmol /l) |
94,0±4,5 |
85,0-103,0 |
67,0±4,0 |
59,0-75,0 |
Р < 0,01 |
|
Indirect bilirubin (mcmol /l) |
51,0±7,8 |
35,4-66,6 |
31,0±6,8 |
17,4-44,6 |
Р > 0,05 |
|
Thymol sample (un.) |
14,5±1,8 |
10,9-18,1 |
9,1±0,6 |
7,9-10,3 |
Р < 0,05 |
|
Prothrombin index (%) |
66,0 ±5,3 |
55,4-76,6 |
78,3±6,4 |
65,5-91,1 |
Р > 0,05 |
|
Total protein (g/l) |
72,3±5,6 |
61,1-83,5 |
69,5±6,2 |
57,1-81,9 |
Р > 0,05 |
|
Albumin (%) |
41,5±3,0 |
35,5-47,5 |
52,5±4,6 |
43,3-61,7 |
Р < 0,05 |
|
Cholesterol (mcmol /l) |
4,8 ±0,2 |
4,4-5,2 |
5,1 ±0,3 |
4,5-5,7 |
Р > 0,05 |
|
Alkaline phosphatase (un/l) |
156,0±9,7 |
136,6-175,4 |
110,0±5,3 |
99,4-120,6 |
Р < 0,01 |
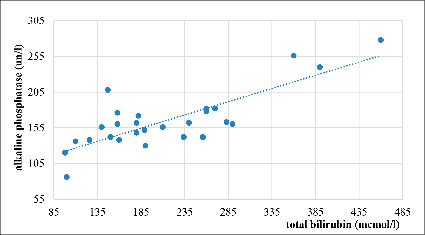
Fig. 4. Correlation between total bilirubin and alkaline phosphatase in severe AVHE, r=0,81 (n=28)
Figure 5 shows a moderately negative correlation between the levels of total bilirubin and albumin concentrations in severe AVHE (r = -0,36, n = 28). These findings may indicate a deterioration in synthetic liver function due to the presence of a bilirubin-induced syndrome.
Figure 6 shows an extremely weak negative correlation between the level of total bilirubin and the activity of alkaline phosphatase in patients with severe AVHA (r = -0,08; n = 17), which probably reflects the distinctive pathophysiological mechanisms of liver damage in this form of infection.
Figure 7 shows a weak negative correlation between the level of total bilirubin and albumin concentration in severe AVHA (r = -0,33; n = 17), which may also indicate a slight decrease in synthetic liver function against the background of bilirubinemia in this nosology.
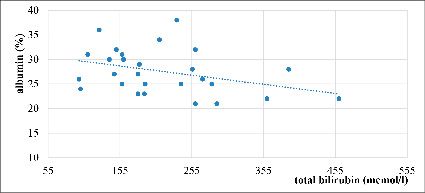
Fig. 5. Correlation between total bilirubin and albumin in severe AVHE, r= – 0,36 (n=28)
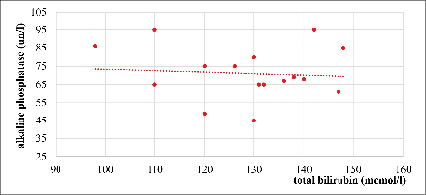
Fig. 6. Correlation between total bilirubin and alkaline phosphatase in severe AVHA, r= – 0,08 (n=17)
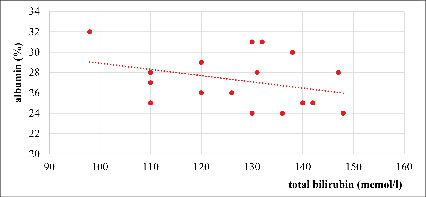
Fig. 7. Correlation between total bilirubin and albumin in severe AVHA, r= – 0,33 (n=17)
The results of the correlation analysis show a difference in the nature of pathogenic processes in severe cases of AVHE and AVHA, and can be used to develop diagnostic and prognostic strategies for patients with these conditions.
Conclusions
A comparative assessment of clinical and laboratory characteristics revealed significant differences between acute viral hepatitis E and acute viral hepatitis A in adult patients. AVHE mainly affected older age groups and was characterized by a more severe course, an extended prodromal period, a high incidence of severe jaundice and a more pronounced cholestatic syndrome compared with AVHA.
Laboratory data from patients with AVHE indicated more severe liver dysfunction, as evidenced by higher levels of bilirubin, ALT, alkaline phosphatase, and a decrease in albumin concentrations.
These findings emphasize the need for increased clinical vigilance for hepatitis E in endemic areas and the optimization of diagnostic approaches, particularly among adult patients.
Acknowledgements
The author expresses gratitude to the staff of the US Centers for Disease Control and Prevention (CDC), the Federal State Budgetary Scientific Institution I.I. Mechnikov Scientific Research Institute of Vaccines and Serums (Russia) and the National Institute of Public Health (Ministry of Health of the Kyrgyz Republic) for methodological assistance in conducting serological studies and advisory support within the framework of the program of epidemiological surveillance of acute viral hepatitis in the Kyrgyz Republic. Special thanks are also expressed to the doctors and medical staff of Osh, Jalal-Abad and Batken regional clinical hospitals for their assistance in collecting clinical material and organizing patient monitoring.

