Seafood processing is one of the critical industries that brings a lot of foreign currency to our country. According to statistics in 2021, Vietnam currently has 815 industrial-scale seafood processing establishments eligible for export and over 3,200 small-scale processing facilities for domestic processing and consumption in operation, with a total processing capacity of up to 6 million tons of raw materials/year, creating over 2.1 million tons of products/year. However, along with the achievements, the seafood processing industry also causes many environmental problems because of its nature and waste composition. In particular, wastewater from seafood processing is highly salinized, containing organic matter, solid waste, and grease [1]. On the other hand, due to the lack of fresh water, many processing plants near the sea use seawater to wash the machinery system, further increasing the salinity of wastewater.
Treating salty wastewater by biological methods is not easy; the COD removal efficiency could be higher due to the side effects of salt on the microflora. In salty or highly salty wastewater environments, microorganisms lose their activity because of plasmolysis, making traditional wastewater treatment biotechnologies ineffective [2]. However, this is a very environmentally friendly solution for worldwide water pollution problems. Therefore, isolating microbial strains in salt-contaminated wastewater, which can remove organic matter and nutrients in salt-contaminated organic sewage, is a right and promising direction.
Therefore, this research is carried out to initially select native strains of bacteria with good halotolerance, producing highly active extracellular enzymes. The goal is to create biological products for wastewater treatment of marine and aquatic products.
Materials and methods of research
The strains of halotolerant bacteria were isolated from wastewater samples of Da Nang Seafood Joint Stock Company – Seafish Corp, which is located at 19 Van Don, Nai Hien Dong, Son Tra, Da Nang.
Methods for isolation and preliminary identification of Bacillus bacteria
Put 10 ml of aquatic wastewater in a triangle jar, add 90 mL of physiological saline (NaCl 9‰), and heat in a thermostatic tank at 80◦C for 10 minutes. Then, dilute the sample at 10-1 to 10-6 concentrations. Aspirate 100 μL of sample in small diluted concentrations onto a jelly disc containing LB medium and incubated at 30ºC. After two days, when colonies appear, colonies are selected that are loose and differ in color, shape, and size [3]. Isolated Bacillus bacteria are preliminarily identified by physiological and biochemical characteristics of Bacillus bacteria according to Claus & Berkeley 1872 with biochemical tests such as Oxidase, Catalase, Voges Proskauer (VP), the ability to use Citrate, the ability to break down Gelatin, the ability to use glucose sugars, sucrose, growth characteristics at 5ºC, 42ºC, and concentration of 10% NaCl.
Methods for determining the halotolerance of isolated Bacillus strains
The growth of bacterial strains was observed while changing the salinity level of the screening isolation medium under the following conditions: The liquid LB medium was designed with salt concentrations of NaCl: 1; 2; 4; 6; 8%, followed by 5% bacterial proliferation. Shaking culture at 150rpm, 37°C, pH 7 measured OD600nM to determine bacterial density after 18 hours [4].
Methods for determining the extracellular protease enzyme generation of Bacillus strains
The ability of bacterial strains to produce proteases was determined by perforation . The proteolytic ability of bacteria is evaluated by its ability to create a resolution ring on the medium, calculated by the formula: D – d
Where: D is resolution ring diameter, d is jelly hole diameter
Methods for determining the protease activity of selected strains of Bacillus
The protease activity of the bacterial strain was determined by the Sigma method with casein substrates [5].
Identification of Bacillus bacteria by PCR technique and 16S rRNA gene sequencing
The DNA of Bacillus bacteria was extracted using a 3% CTAB. The DNA extraction product was then amplified by PCR reaction with the generalized primer pair 27F/1492R with the following nucleotide sequences: 27F:5’ AGA GTT TTC TTC AG 3’ and 1492R: 5’ GGC TAC CTT GTT ACG ACT T 3’ targeting the 16SrRNA gene fragment. The steps in the PCR reaction are as follows: Step 1: 94°C for 5 minutes; Step 2: 94°C for 1 minute; Step 3: 55°C for 1 minute; Step 4: 72°C for 2 minutes; Step 5: Repeat step 2 for another 34 cycles; Step 6: 72°C for 7 minutes and step 7: 4°C for an indefinite time. The composition of a PCR reaction with a total volume of 25 μL is as follows: 12.5 μL Go Taq Green Master Mix, 0.5 μL downstream primer (10 μM), 0.5 μL reverse primer (10 μM), 10.5 μL of DNA-free water and 1 μL of pure DNA. PCR products are sent for sequencing at Fist base company (Malaysia). The sequencing results were compared and traced on the World Genome Bank at the website (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine the species levels of the two strains of bacteria surveyed.
Method for investigates several factors affecting the protease generation capacity of selected Bacillus strains
The culture of selected Bacillus bacteria in medium (LB) has a breeding rate of 5%, pH = 7, temperature 37°C, and a shaking rate of 200 rpm. Investigate several factors affecting protease generation, including:
- Initial pH: The environmental pH survey is 5; 5.5; 6; 6.5; 7; 7.5; 8
- Nitrogen sources: environments with added nitrogen sources (KNO3, NH4Cl, NH4NO3, casein, Tryptone) replace 0.5% high yeast in LB medium.
Data processing methods
The data was processed for descriptive statistics using Microsoft Excel. Data processed using the ANOVA ONEWAY method has a difference of P<0.05 and a reliability of 95%.
Results of the research and discussions
Isolation of bacterial strains from seafood processing factory wastewater
From three wastewater samples, the seafood processing plant was isolated from the LB medium, and four strains of bacteria were obtained. The strains were purified on the LB medium, and then colony morphology, cell size morphology, and biochemical physiological properties were tested. After comparing Bergey’s (1872) bacterial classification key [6], four preliminary strains were preliminarily concluded to belong to the Bacillus genus and were denoted from B1 to B4 (Table 1, Figure 1).
Halotolerance of bacterial isolates
Figure 2 shows the different adaptations of four strains of Bacillus spp. (B1, B2, B3, B4) with salinities ranging from 1% to 8%. In environments containing 1% and 2% NaCl, all four strains of Bacillus spp. grow very well. This may be because the salinity of seafood processing plant wastewater is within 3%, so it is easy to understand that strains can adapt to the salinity of 2%. However, bacterial strains grow weaker in environments with higher salt concentrations of 6%. At 8% salt concentration, only three strains, B1, B2, and B3, can live, but their growth rate is significantly reduced. This result is also consistent with the study of Le Hung Anh et al. 2020, which investigated the halotolerance of bacterial strains from aquatic wastewater.
Table 1
Characteristics of bacterial strains isolated from seafood processing plant wastewater
|
Norms |
B1 |
B2 |
B3 |
B4 |
|
|
Colony morphology |
Milky white, dry, adheres on agar |
White, serrated margin |
Milky white, rough, dry, rounded, wrinkled dry surface |
Milky white, with knobs in the middle, spreading edging |
|
|
Shape |
Rod |
Rod |
Rod |
Rod |
|
|
Cell size (μm) |
0,9-1,0 x 2.7-3.1 |
0,3-0,8 x 1.3-2.6 |
0,2-0,5 x 1.2-1.9 |
0,7-1,1 x 1.9-3.1 |
|
|
Gram stain |
+ |
+ |
+ |
+ |
|
|
Spore location |
middle |
middle |
middle |
eccentric |
|
|
Oxidase |
+ |
+ |
+ |
+ |
|
|
Catalasa |
+ |
+ |
+ |
+ |
|
|
Idol |
- |
- |
- |
- |
|
|
VP |
+ |
+ |
+ |
+ |
|
|
Movable |
+ |
+ |
+ |
+ |
|
|
Fermentability |
Glucose |
+ |
+ |
+ |
+ |
|
Mannitol |
+ |
+ |
+ |
+ |
|
Note: (+) positive; (-) negative
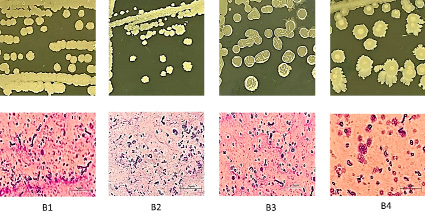
Fig. 1. Colony morphology and cells of bacterial strains isolated from seafood processing plant wastewater
The author found that 45 strains grew well in environments containing 1% NaCl. Of these, only 33 strains could grow in higher environments with 3% NaCl after 24 hours of culture. At higher salt concentrations, their growth rate decreases, and after 45 hours, only 14 strains and 12 strains can live in environments with salinities of 5% and 7%, respectively. High salinity directly impacts the transfer of ions into and out of the cell membrane, affecting the osmotic pressure of the membrane. Only three strains, 1B1, 2A2, and 3A5, survive at 7% salinity but have the lowest maturity [7]. The high halotolerance of 04 strains of Bacillus spp. may be related to the low intracellular salt concentrations of these strains, and they maintain an osmotic balance between their cytoplasm and the external environment by accumulating high concentrations of various osmotic solutes.
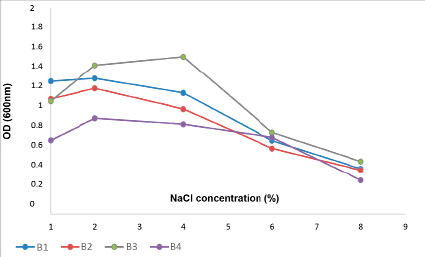
Fig. 2. The growth of B1, B2,B3, B4 on mediums with different concentrations of NaCl salts
In addition, halophilic bacteria such as Halomonas and Halobacterium synthesize ectoine and L-glutamate to survive salinity-stressed conditions. Some halophilic bacteria include Bacillus, Pseudomonas, Aeromonas and Zymomonas use polyol compounds such as sorbitol, arabitol, glycerol, and mannitol for osmotic adaptation under salt deficiency conditions. In 2020, Guizhong Zhou, Xitong Wang also suppose permeability through the relative membrane of the strain Bacillus cereus remains stable under conditions of high salt stress condition due to glycine and proline play essential roles in maintaining cellular permeability. This strain’s protein and soluble sugar content was increased at higher salt concentrations [3].
Determination of protease pathogenesis of selected bacterial strains
The results of the study showed that all four strains of isolated Bacillus spp. can all produce extracellular proteases expressed in casein hydrolyzed ring diameters (1%) on agar dishes. In particular, strain B3 has the highest protease enzyme capacity, with a resolution ring diameter of 18.3 ± 0.577 mm, while the lowest is strain B1, with only 13.3 ± 1.155 mm (Table 2). This result is also similar to Nguyen Thi Kim Co et al. (2019) research [8]. According to the author, strains B6, B8, B9, and B14 give the highest protease enzyme generation with a resolution ring diameter of 14.33, respectively, 18,33, 16.67, and 18.67 mm.
Table 2
Protease activity of 4 isolated strains of Bacillus spp.
|
Strains |
Hydrolysis ring diameter (mm) |
|
B1 |
12.7 ±1,000c |
|
B2 |
16,3 ± 0,577ab |
|
B3 |
18,3 ± 0,577a |
|
B4 |
13,3 ± 1,155c |
Note: Different letters on the same column indicating a difference have a statistical significance level of the sample mean with p < 0.05.
Our results are 4 mm lower than those of G. Pant et al. (2015) [9]. The Bacillus subtilis strain in G. Pant’s study once recorded a protein hydrolysate ring diameter of 22 mm. While Laili N. and Antonius S. [10] conducted proteolytic ring measurements in Bacillus sp. isolated strains in soil, strain conclusions have had a giant hydrolysis ring of 15 mm.
B3 strain identification results
Since the B3 strain has a 99.05% homologous 16S rRNA gene region sequence with Bacillus velezensis YA215, it can be concluded that the bacterial strain isolated from seafood processing plant wastewater is a Bacillus velezensis species (Figure 3).
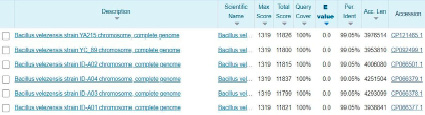
Fig. 3. B3 bacterial homologous sequence search results
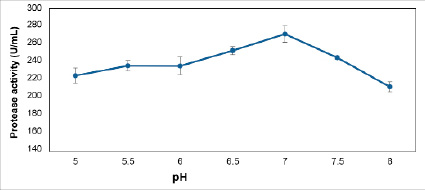
Fig. 4. Effect of pH concentration on strain’s ability to produce protease enzymes of Bacillus velezensis B3
Identify some factors affecting the protease activity of Bacillus velezensis B3 strains
Effect of pH concentration on protease generation capacity of Bacillus velezensis B3 strain
The result showed that the Bacillus velezensis B3 strain can grow and develop in both acidic and basic environments. Protease activity varies with the pH values surveyed. When the pH of the medium increases from 5 to 6.5, the protease activity tends to increase gradually, with relative activity increasing by 206.27 U/mL to 241.25 U/mL. The highest activity achieved at pH 7 was 264.39 U/mL (Figure 4).
Protease enzyme activity decreases significantly at pH ranges higher than the optimum pH. The activity is only 190.69 U/mL at pH 8. This result is similar to the survey of Tran Hong Thi et al. 2012, all ten strains of Bacillus give the best enzyme activity at pH 7. The most potent protease activity at this value is M5 (0.87 U/mL), and the weakest is M1 strain (0.68 U/mL) [11]. Prihanto et al. also consistent with above results, the Bacillis subtisllis UBT7 strain gives peak activity at pH 7.0 [12].
Meanwhile, Bacillus subtilis Bs04 strains have the highest enzyme activity recorded at pH 7.5 at 0.434 U/mL–proteases from B. flexus APCMST strains and Bacillus sp. APCMST-RS7 also exhibits the most vigorous activity at pH 8 and 50°C. Protease yields vary considerably with pH, which may be due to other components of the environment and their combined influence on the metabolism of bacterial species [13].
Effect of nitrogen source on the extracellular protease production capacity of B. velezensis B3
In the investigated nitrogen sources, organic nitrogen sources were better than inorganic sources at stimulating protease generation by B. velezensis B3 strains. Casein caused the highest increase in protease activity (437.1 U/mL), higher than the control sample of 181.09 U/ml (Figure 5).
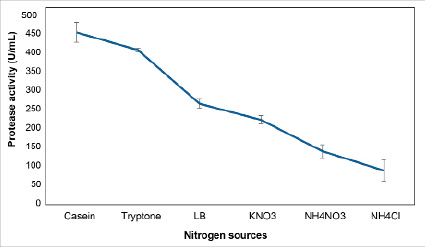
Fig. 5. Effect of the addition of nitrogen sources on protease activity in Bacillus velezensis B3 strain culture
Many researchers have reported that organic nitrogen sources are better suited to Bacillus spp. for protease enzyme growth and production than inorganic sources. It has been reported that peptone, casein, skim milk, yeast extract facilitate maximum protease production by Bacillus spp [14]. In study of Pant G et al., the presence of galactose and peptone in the medium enhanced enzyme production by 0.5% when compared with other carbon and nitrogen sources [9].
Adding Tryptone to LB medium to replace 0.5% yeast extract also contributed to an increase in protease activity (392.4 U/mL). However, the protease activity was not higher than that of the casein ( 437.1 U/mL) supplement formulation but still higher than that of the control sample, 136.39 U/mL.The salts KNO3, NH4Cl, and NH4NO3 did not show the ability to increase protease activity, which reduced protease activity; the content was 40.94 U/mL, 120.72 U/mL, and 169.78 U/mL compared to the control (LB medium).
Thus, casein is the best source of nitrogen for Bacillus velezensis B3 bacteria strains to biosynthesize protease enzymes.
Conclusions
From wastewater samples taken from Da Nang Seafood Joint Stock Company – Seafish Corp, four strains of bacteria of the Bacillus genus, symbols B1-B4, have been isolated. The research conducted surveys on salt tolerance and the ability to produce extracellular protease enzymes, and the B3 strain with the highest activity was selected. The results of identification of the B3 strain by sequencing the 16S rRNA gene showed that the B3 strain belongs to the species Bacillus velezensis. The research determined that pH 7 media and the addition of a nitrogen source, casein, gave the highest protease enzyme activity with enzymatic activity of 437.1 U/mL.

