Multiple sclerosis is the world’s most common chronic neuroinflammatory and neurodegenerative disease of the central nervous system, characterized by the appearance of demyelination foci in the CNS and damage to axons, which most often occurs in a relapsing form [1], when the clinical course is characterized by acute neurological symptoms (relapses) separated by periods of relative calm (remissions). Multiple sclerosis, which usually affects patients between the ages of 20 and 40, ranks first among the causes of disability among the youth of the USA and Europe [2]. The pathogenesis of the disease is based on complex and dynamic interactions between the body’s immune system, glia (myelin-producing oligodendrocytes and their precursors, microglia and astrocytes) and neurons, which lead to demyelination of the nerve fibers. Typical acute neurological symptoms include (but are not limited to) visual, sensory, and motor impairments. Recovery from relapses may be incomplete, and disability usually increases with time [1].
Etiology of multiple sclerosis
The etiology of multiple sclerosis still remains unspecified. The main agents responsible for the development of MS are exogenous (tobacco smoke), environmental (environmental conditions) and genetic (congenital malformations) factors [3], and dishormonal disorders in the human body or the microbiome can also influence the onset of the disease [4].
Regarding the environment, the main risk factors include geographic latitude, which probably reflects seasonal changes in sunlight exposure that affect vitamin D levels or pathogens prevalent in these regions, although a genetic contribution is also possible. Tobacco exposure and obesity are also associated with an increased risk of developing multiple sclerosis. A virus (Epstein-Barr virus), bacterium, or other environmental toxin can trigger an immune response in genetically predisposed people [4]. Viruses other than Epstein-Barr viruses have been proposed as potential causes of MS or MS-related disease activity, but none have been conclusively proven. Some of them may act as molecular mimics, while others may interfere with the mechanisms that normally limit self-reactive cells. Differential susceptibility is reflected in the mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), so that specific myelin antigens are required to induce EAE in different strains of mice. The microbiome can also strongly influence the propensity to develop EAE (especially in genetically predisposed strains with transgenes for myelin recognition by B and T cells), and evidence of a similar phenomenon in MS patients is beginning to emerge. In general, the question of which genetic polymorphisms and environmental influences increase the risk of developing multiple sclerosis remains the subject of intense research.
The role of the autoimmune component in the development of MS
It is believed that MS is an autoimmune disease. This statement is controversial because when comparing MS with other autoimmune diseases such as neuromyelitis optica (ONM) (Table 1).
Table 1
Revised criteria for a disease to be considered autoimmune: comparative characterization of neuromyelitis optica and multiple sclerosis [5]
|
Criteria for an autoimmune disease |
Opticoneuromyelitis |
Multiple sclerosis |
|
Immune response to a specific autoantigen in all patients |
Aquaporin 4 |
Many antigens have been described that may be absent in patients |
|
Lesion reproducibility after administration of autoantibodies or T cells in models |
Exacerbation of the experimental autoimmune encephalomyelitis model after adaptive transfer of neuromyelitis optica Abs |
|
|
Lesion induction by antigen immunization in animals |
EAE model: induction by myelin oligodendrocyte glycoprotein, proteolipid protein, myelin basic protein, and reactivated CD4+ T-Cells |
|
|
Isolation of autoantibodies or T cells from the lesion or serum |
Antibodies to antiporin 4 |
|
|
Autoantibody titers or T cell levels associated with disease activity |
Higher antibody titers during relapse than during remission |
|
|
Autoimmune disorders or autoantigens associated with the disease |
Sjögren’s syndrome, SLE |
No association in population-based cohort studies |
|
Immune uptake by the purified autoantigen eliminates pathogenic autoantibodies or T cells. |
||
|
Decrease in autoantibodies or T cells associated with clinical improvement |
With plasmapheresis |
With plasmapheresis |
it becomes clear that MS does not meet the full definition of an autoimmune disease. While VUI meets six of the eight criteria for an autoimmune disease, MS only meets two.
However, there is evidence for other proposed criteria, but the evidence for their validity is conflicting. For example, there are studies focusing on the measurement of progenitor T cells before and during clinical exacerbations of multiple sclerosis [6]. However, none of these studies found significant differences with prevalence in healthy controls, or were proven using other patient populations [7]. From this we can conclude that there is not enough data for us to definitively conclude that MS is an autoimmune disease.
The main links in the pathogenesis of MS
As is clear from the definition, MS is an inflammatory, neurodegenerative, demyelinating disease. Therefore, the main links in the pathogenesis will be: inflammation (autoimmune process), demyelination (destruction of myelin) and neurodegeneration (damage to nerve fibers). These mechanisms will be discussed in more detail below.
Inflammation in MS
In the case of multiple sclerosis, inflammation is caused by self-reactive T-cells that have penetrated the blood-brain barrier, namely T-helper types 1 and 17. These autoreactive T cells interact with adhesion molecules located on the endothelium of CNS venules and, together with antibodies and monocytes, overcome the destroyed BBB with the help of proteases (for example, matrix metalloproteinases) and chemokines (interleukin-17 (IL-17), interferon-γ ( Ifn-γ) and tumor necrosis factor alpha (TNFα)), opening the way for other cells to enter the CNS.
Further, autoreactive T-helpers 1 and 17 t hat have entered the CNS are reactivated, recognizing target antigens [4]. B-cells that enter the CNS continue their maturation, turning into plasma cells, producing anti-myelin antibodies that bind to their antigenic targets (myelin), in turn triggering the activation of the complement system with the formation of a membrane attack complex that damages myelin sheaths and causes demyelination. Monocytes become activated macrophages, which in turn secrete reactive oxygen species and nitrous oxide (NO), phagocytizing the degraded myelin in the CNS. CD8+ cytotoxic T-lymphocytes are also involved in the process, which not only cause lysis of the oligodendrocytes themselves and demyelination, but also the death of axons, that is, neurodegeneration (Figure).
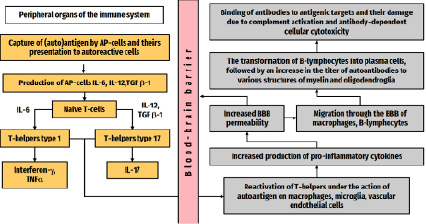 Main stages in the pathogenesis of inflammation in MS
Main stages in the pathogenesis of inflammation in MS
At the same time, under the influence of cytokines, CD4+ T cells proliferate into T-helper type 2 cells and secrete anti-inflammatory cytokines and transforming growth factor β, which suppress the immune response and further damage to myelin sheaths and oligodendrocytes [4].
Role of B cells in MS
Although MS is generally considered a T-cell mediated disease, a growing body of evidence supports a pathogenic role for B cells, including the frequent observation of intrathecal immunoglobulin production in patients with multiple sclerosis, the identification of antibodies that respond to specific myelin antigens in multiple sclerosis, a pathological picture of multiple sclerosis characterized by antibody-associated demyelination and detection of B-cell follicles in the meninges of patients with secondary progressive multiple sclerosis.
Research shows that B cells influence the development and progression of MS by acting on autoantigens. In addition, humoral antibodies have been reported to cause tissue damage when they bind to brain cells and interfere with complement factor function. More recently, leptomeningeal B cells have been found to cause neuronal degeneration and demyelination [8]. In addition, B cells can deplete anti-CD20 antibodies, causing MS relapse and further neurological damage. However, target antigens in the development of MS remain the subject of debate and research. Despite this, B cells make a significant contribution to the development and progression of MS [9].
Neurodegeneration in MS
At present, we have three possible mechanisms for the neurodegenerative process in MS. Neurodegeneration is the death of nerve cells, which at the final stage leads to a complete stop in the transmission of the nerve impulse. In MS, it develops independently of autoimmune inflammation and is not directly related to it.
1) In the blood plasma, brain and cerebrospinal fluid of patients with multiple sclerosis, an increased content of glutamate is found [10], the data are confirmed by studies that confirmed the presence of processes of axonal damage and death of oligodendrocytes in the brain of patients with multiple sclerosis and in research models with experimental autoimmune encephalomyelitis (EAE) [11]. Glutamate (glutamic acid) performs the function of an excitatory neurotransmitter in the CNS, but with the accumulation of this substance in large quantities (if the concentration of glutamate in the synapse is exceeded by more than 1 mmol, apoptosis processes will be triggered) leads to stimulation of the NMDA receptors of other neurons, after which these neurons receive calcium, and this already leads to the launch of a number of pathological mechanisms and the death of the neuron. Thus, one of the mechanisms of neurodegeneration is the excitotoxicity of glutamate.
2) Another mechanism may be the redistribution of ion channels and a change in their permeability in the axons of neurons. These phenomena can lead to disruption of the ionic balance, which ultimately results in damage, and in the worst case, death for the axon.
3) The third mechanism of neurodegeneration can be a shift in the balance of remyelination factors, which are necessary for the further survival of oligodendrocytes and neurons in the brain.
Mechanisms of demyelination in MS According to the results of studies by C.F. Lucchinetti and his associates (2000) identified 4 demyelination mechanisms in MS: phage-associated, antibody-induced, distal oligodendropathy, primary oligodendrocyte degeneration time, the differences in the flow between them completely disappear) [12].
Factors affecting the course of multiple sclerosis
1) Pregnancy as an inducer of immunological tolerance. The results of the Pregnancy in Multiple Sclerosis study, which included 254 pregnant patients with multiple sclerosis, showed that the recurrence rate of multiple sclerosis decreased during pregnancy, mainly during the last trimester, while the recurrence rate increased 3 months after delivery and was equal to the recurrence rate before pregnancy [13]. Therefore, we can say that in pregnant patients with MS, the condition improves significantly, but after the onset of the postpartum period, the course of the disease worsens, this may be due to a drop in estrogen levels. Estrogens and other sex hormones activate immunological transformation during pregnancy by switching T-helpers mainly to Th2 (anti-inflammatory effect) instead of Th1 (pro-inflammatory effect), while immunomodulation is restored after delivery [14]. Pregnancy hormones contribute to a sharp increase in the number of regulatory T- and B-lymphocytes, which weaken the development of the immune response and reduce the risk of fetal rejection by the mother’s body [15].
2) The negative impact of smoking on the course of the disease. Smoking not only increases the risk of multiple sclerosis [16], it also negatively affects the process of MS treatment itself. Since smoking increases the risk of developing neutralizing antibodies against biologics that are widely used in MS therapy (natalizumab [17] and interferon β [18]), therefore, the treatment will be ineffective. Also, the process of tobacco smoking itself is associated with worse prognosis in MS [19].
3) Theory of the impact of low temperatures. This study provides a critical understanding of the pathogenic mechanisms of neuroinflammation and may lead to the development of future preventive and therapeutic approaches to multiple sclerosis and other autoimmune diseases. To test their hypothesis, the scientists placed experimental models (mice with EAE) in a relatively cold environment, around 10°C. This was preceded by a period of acclimatization of animals with a gradual decrease in ambient temperature [20]. Doron Merkler, professor in the Department of Pathology and Immunology and the Center for Inflammatory Research at the UNIGE Faculty of Medicine (and co-author of the paper), reports that after a few days there was a clear decrease in the clinical severity of the disease, as well as the degree of demyelination in the central nervous system. The organism of animals did not experience difficulties in maintaining a normal level of body temperature; but, in particular, the symptoms of movement disorders have sharply decreased – starting with complete paralysis of the hind legs to a slight form of paralysis of the muscles of the tail.
This study presents a systematic review of changes in immune cells in bone marrow and blood in response to exposure to cold at steady state and shows that lower ambient temperature reduces neuroinflammation. Cold-induced changes in gene expression were accompanied by phenotypic and functional changes in monocytes. Decreased expression of major histocompatibility complex II and its associated pathways in these cells was also accompanied by a decrease in antigen-presenting capacity after various inflammatory stimuli. This cold-induced modulation of monocytes resulted in decreased priming of autoreactive T cells, resulting in attenuation of CNS autoimmune disease (Table 2).
As a result, T cells (a type of immunocompetent cells that play an important role in the pathogenesis of autoimmunity) become less active. By forcing the body to increase its metabolic rate to maintain a constant body temperature, low ambient temperatures take resources away from the immune system. This leads to a decrease in the number of potentially dangerous immune cells and, therefore, alleviates the course of the symptoms of the disease.
As a result of the study, the experimenters came to the conclusion that cold temperature modulates the immunological and metabolic phenotype of monocytes, and also contributes to an energy compromise between metabolic adaptation and autoimmunity in mice, which leads to a pronounced decrease in the clinical manifestations of demyelination and neurodegenerative processes in the CNS.
Table 2
Results of the effect of cold temperature on experimental models of RS
|
Bone marrow |
Lymph nodes |
Central nervous system (CNS) |
Experimental mice with EAE |
|
|
Room temperature (22-23°C) |
Expression of MHC II by monocytes |
Priming of T cells by monocytes |
Demyelination, neurodegeneration |
Severe paralysis |
|
Exposure to cold temperature (10°C) |
Decreased expression of MHC II by monocytes and reduced neuroinflammation |
Decreased priming of T cells by monocytes |
Decreased tissue damage in the CNS |
Marked improvement in symptoms |
Conclusion
The etiology of multiple sclerosis is unknown, but it is likely due to the complex interaction between environmental and genetic factors and the immune system. The clinical manifestations and course of multiple sclerosis are extremely variable, but most patients develop disability over time. To optimize the results of treatment, clinicians should be familiar with the etiology, pathogenesis, risk factors and processes affecting the course of the disease.

