The European classification of retinopathy was proposed in 1991 by E. Kohner and M. Porta. This classification is very simple and easy to use in practice, and it can be used to clearly define the stability of the retinal lesion. Importantly, by using this classification, it is possible to estimate precisely at what stage the retinal damage occurred and the need for correction and treatment. According to this classification, there are 3 main forms of retinopathy: non-proliferative, preproliferative and proliferative, the latter being characterized by 2 types of proliferation: vascular and fibrous. Most authors consider the duration and type of diabetes mellitus, glucose compensation, blood pressure, and renal status to be the main risk factors for retinopathies.
Studies have shown that hyperglycemia is the underlying cause of retinopathy, which can damage cells and influence their death. The death of endothelial cells in retinal vessels contributes to the disturbance of blood circulation in the retina through the deposition of fibrin, which is one of the factors of clot formation, which can lead to the process of cell proliferation, which in turn leads to thickening of the basal membrane [1].
The main risk factors for retinopathy include prematurity and low birth weight. Studies have identified various factors that exacerbate the overall systemic status of preterm infants (intraventricular haemorrhage, bronchopulmonary dysplasia, anaemia, infection, and glucocorticosteroid drugs). The use of inadequate oxygen therapy regimens for preterm infants plays an important role in the severity of retinopathy and is a key risk factor for severe retinopathy.
Understanding the mechanisms of development of pathological processes in alterations of the structural elements of the vitreous body is unlikely without studying the patterns of morphogenesis and the signal pathways that provide them. The processes of development of transparent media of the eye have been studied. The periods of restructuring and involution of the eye structures involved in the formation of transparent eye media associated with angiogenesis and devastation of blood vessels have been established. The concept of the general origin of cells involved in the secretion of crystallins and the general patterns of signal interactions of cell ensembles of cells that form the visual cortex of the brain, retina, vitreous body, cornea, secreting crystallins are proposed [2].
Relevance
In order to better understand in vivo the numerous pathways and mechanisms leading to the pathogenesis of diabetic retinopathy and its complications, such as diabetic macular edema, to create the potential for more individual targeted treatment, it is necessary to study cellular interactions in the system of structures of the developing eye during human ontogenesis. Persistent hyperglycemia leads to the activation of many cellular pathways involved in the pathogenesis of diabetic retinopathy (DR), resulting in increased inflammation, oxidative stress, and vascular dysfunction. DR, however, there is growing evidence that both inflammation and neurodegeneration occur in human diabetes even before clinical signs of DR develop [3]. Despite the presence of refractive lenses in the complex and compound eyes of many invertebrates, relatively little is known about their crystallins. The revealed numerous refractive structures, which have developed in the eyes of invertebrates, noticeably contrast with the limited information on their protein composition, which indicates its insufficient knowledge. One of the first signs of inflammation in diabetes is the activation of retinal glial cells (RGC), which, with the appearance of inflammatory mediators, growth factors in the retinal layers, represent an in vivo marker of microglial activation accompanied by early loss of nerve cells, confirmed the hypothesis that neurodegeneration occurs at an early stage in both type 1 and type 2 diabetes. However, these mechanisms can be understood only on the basis of exhaustive ideas about the mechanisms of development of the human eye and the involution of its temporal structures [4].
Purpose of research
Explore induction of the development of transparent environments of the human eye.
Material and methods
The study was carried out with the permission of the Ethics Committee of the Far Eastern Federal University. The provisions of the Declaration of Helsinki (2000, 2013) have been taken into account and taken into account. Using the material of 15 eyes of human embryos and fetuses, the time and localization of S100B positive cells in the transparent media of the eye were established by the method of immune histochemistry. The preparations are stained by the classical method with hematoxylin and eosin. Langerhans cells were detected by immunohistochemical method followed by analysis on a microscope Olympus-Bх-52.
Research results and their discussion
The development of eye structures was studied to clarify the role of neuroglial migrants from the wall of the optic cup and ectomesenchyme located between the cerebral vesicle and the ectoderm of the head end of the embryo in the tissue organization of the vitreous body, lens and cornea [5]. Using the material of 15 human embryos and fetuses by the method of immune histochemistry, the timing and localization of S100B positive cells in the transparent media of the eye, migrants not only from the ectomesenchyme surrounding the forming optic cup on the posterolateral surfaces, but also from the neural retina, were established (figure). At week 5, not only active migration of precursor neuroglial cells, which form a matrix for the formation of the internal bipolar and ganglionic layers, occurs from the inner wall of the optic cup, but also its eviction outside the optic cup wall in the direction of the vitreous body, lens and cornea. Ectomesenchyme, which is the basis for the formation of a common rudiment for the vascular and fibrous membranes of the eye, serves as the main source of scleral development. Differentiation of ectomesenchymocytes goes in the direction of fibroblasts synthesizing connective tissue of the opaque sclera, in which active vasculogenesis occurs [6].
Neuroglial migrants from the retina populate the forming structures of the transparent media of the human eye and differentiate into a special type of fibroblasts that, like the neuroglia of the retina and visual cortex, are capable of secreting an extracellular matrix consisting of crystallins and a basic substance that inhibits the germination of blood vessels. Involution of the vascular capsule of the lens and the hyaloid basin of the vitreous body is associated with this process [7]. Differentiation of neuroglia inhabiting the transparent media of the eye and the outer neuronal retinal layer occurs earlier than macroglia in the bipolar and ganglionic layers, and the common origin of neuroglial derivatives explains the absence of their own blood vessels in the photoreceptor layer of the retina and transparent media of the human eye [8].
The physically transparent cornea, lens and vitreous humor are opaque from a histophysiological point of view and, therefore, cannot directly transmit light to the retina. The developing lens has 2 sources of development: ectoderm, as a source for the capsular epithelium and neuroglial, for the stroma. Crystallins make up 80-90 % of the water-soluble proteins of the clear lens. Crystallin recruitment occurs through changes in gene regulation leading to high lens expression. These diverse proteins are responsible for the optical properties of the lens and have been derived from metabolic enzymes and stress proteins [9]. The development of retinal vessels is a complex process that has not yet been fully understood. Most of the research in this area has focused on astrocytes and the structures they form in the inner wall of the optic cup, a period that precedes endothelial cells and angiogenesis in the retina. However, in humans and dogs, astrocyte migration follows the development of blood vessels, suggesting that other cell types are able to induce this process. One of these cell types is the ganglion cell, which differentiates to form blood vessels and is located adjacent to the primary vascular plexus of the retina. The authors’ data indicate that neuroglial migrants from the retina, as a result of differentiation, then specialize in stromal fibroblasts of the base of the cornea, as well as cell differentions of the vitreous body of the human organ of vision. A single source of transparent structures of the eye is explained and confirmed by the fact that the cornea, lens, vitreous body, neuroglia of the retina and brain contain proteins that are specific to all these structures – crystallins [10]. As known 59 kDa crystallin polypeptide previously observed in octopuses is omega-crystallin and has been identified as aldehyde dehydrogenase. Based on the notion that the same transcription factors (for example, Pax-6, retinoic acid receptors, maf, Sox, AP-1, CREB) regulate different crystallin bead genes, it can be assumed that the common features of lens-specific expression played a key role in attracting a variety of multifunctional proteins such as crystallins. Cellular interactions and histophysiology of fibroblasts of the transparent media of the eye explains the identity of the functions of crystallin secretion. Physiochemical properties and characteristics of crystallins prevent diffusion of light and direct it in one channel [11]. The authors’ data on neuroglial sources of origin of the lens stroma suggest that lens cells may act like Mullerian glia in the regulation of transformed energy flow. Consequently, all structures of the transparent media of the human eye, including the fibers and stromal cells of the cornea, lens, vitreous humor and retinal glia, have the ability to function as components of the universal conductive system of light perception, converting it into another type of energy (presumably into electromagnetic waves or some motor impulse), and only then sending it to the photoreceptors. As a result, we have one-way light conduction with the help of stromal cells of the vitreous structures of the human eye and at the same time the lack of the ability of the retina to identify these cells. That is why the cells located in front of the retina are invisible to photoreceptor cells in normal physiological regeneration. Only those structures that can be identified by this unique conduction system are visible [12].
The structural organisation of the fibrillar vitreous body varies in its different sections. There are optically empty areas, which are limited by membranes up to 20 µm thick. Larger fibers of fibrillary skeleton have predominantly longitudinal direction. Small fibres with cross-section less than 1 µm are arranged obliquely, weaving into larger ones. Backbone fibrils and dissolved collagen, together with hyaluronic acid, contribute to the gel state and play the role of a soft vitreous skeleton. By the orderly arrangement of the fibres the vitreum can be classified as a formed fibrous connective tissue. The fibres of the fibrillary backbone are woven into the optic nerve sheath in the disc area, which provides high contact strength.
Cell proliferative activity changes during the development of the eye and depends on the stage of ontogenesis. During the embryonic and fetal periods, the proliferative activity of the vitreous cells is quite high, but after birth it decreases and then stays at approximately the same level. After 45 years of age, mitotic figures in the vitreous body cells are very rarely observed. Age-related involution of the vitreous body is accompanied by the formation of cavities of various sizes. Involutionary changes also include filamentous destruction, appearing as early as after 20 years of age and increasing after 40 years of age [13].
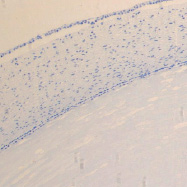
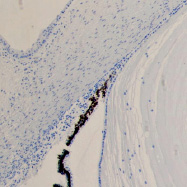
a b
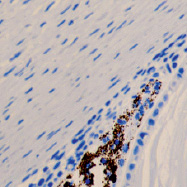
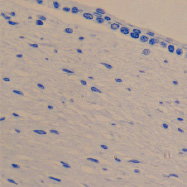
c d
The eye of the human embryo at 12 weeks. Identified: lens, cornea, iris, pigment epithelium (detached); a) anterior pole of the eye; b, c) lateral surface; d) the anterior 3-layered epithelium and the corneal substance proper.Staining with hematoxylin. Microphoto. Magnification: х100 (a), x200 (b), x400 (c, d)
Conclusion
It is known that Muller cells are slightly different in the GFAP retina in the absence of pathology and in diseases of the retina accompanied by detachment. The bases of these cells can be structurally strengthened by the cytoskeleton through intermediate filaments; our data also indicate a critical role for these proteins in the response of Muller cells to retinal detachment and participation in subretinal gliosis. The transparent media of the eye are derived structures of the neuroglial migrants of the human retina. The ability to secrete crystallins unites them into one functional group with the neuroglia of the visual cortex and retina, and also serves as evidence of the influence of the general signaling system in the process of differentiation.
The leading inducers in the development of transparent media of the human eye is the migration of neuroglial cells with subsequent differentiation into structures with the secretion of crystallins that inhibit the vascularization of transparent media of the organ of vision.
The study was financially supported by the International Medical Research and Education Center (Vladivostok, Russia).
Scientific advisers – Doctor of Medical Sciences, Professor G.V. Reva; Doctor of Technical Sciences, Professor A.N. Gulkov.

