At the present stage, it is reliably known that the formation of the oral cavity occurs simultaneously with the formation of other structures of the human embryo. The development of the oral cavity occurs as a result of the interaction of a number of embryonic rudiments and structures. The formation of the primary oral cavity occurs as a result of the formation of an oral cavity called the stomodeum. The bottom of the stomodeum is the pharyngeal membrane, represented by layers of intestinal endoderm and cutaneous ectoderm, adjacent to each other quite closely without connective tissue layers. The stomodeum stops growing at the moment when the pharyngeal membrane breaks, which communicates it with the cavity of the primary intestine. The growth of the oral cavity at this stage is provided by growing outgrowths along the edges of the stomodeum – gill pockets, gill slits and branchial arches. Of particular importance in the formation of the oral cavity are I-III branchial arches. Violation of morphogenetic processes during embryogenesis can lead to developmental abnormalities [1].
The mucous membrane of the gums and hard palate formed as a result of ontogenetic development consists of stratified squamous epithelium and the lamina propria of the mucous membrane. In the stratified squamous epithelium, there are 3 layers: basal, intermediate (prickly), superficial. On a histological section, the basement membrane is represented by a dense network of thin argyrophilic fibers, most of which are closely associated with the processes of the cytoplasm of the cells of the basal layer. The basal epithelium consists of a layer of cylindrical cells with basophilic cytoplasm, large oval nuclei with clear boundaries. The thorny layer is represented by a wide accumulation of several rows of polygonal cells with light cytoplasm and pronounced intercellular bridges. Some of the author admit the possible existence of a pronounced granular layer here, consisting of 2-5 rows of cells. In the thickness of the basal layer, epithelial stem cells are localized, which have a pronounced ability to mitotic division. Due to the formed cells that have entered into differentiation, the epithelial cells of the overlying layers of the epithelium change. The cells of the basal layer take part in the formation of the structural components of the basement membrane. The intermediate layer forms the bulk of the stratified squamous non-keratinizing epithelium. The cells of this layer, moving towards the surface layer, gradually lose their ability to mitosis. The maturation of these cells is capable of forming the surface of the epithelial layer. When studying a histological section of the oral cavity of an embryo, a layer of non-keratinizing epithelium is found, whose cells are capable of producing substances that have an antimicrobial effect. At the same time, the layer of non-keratinizing epithelium is much thicker than the keratinizing one. For these reasons, it can be argued that if the non-keratinizing epithelium in the oral cavity is thinner than the keratinizing epithelium, this can lead to a decrease in local immunity, which in turn will lead to congenital pathology. In the proper gum plate and the mucous membrane of the hard palate, densely located bundles of collagen and a thin network of elastic fibers, characteristic of fibrous connective tissue, are determined, between which fibroblasts, histiocytes, single or in the form of clusters of mast cells and lymphocytes are located [2].
Purpose of our research is the study of the mechanisms of development of the mucous membrane of the human oral cavity in ontogenesis.
Material and methods
The study was carried out with the permission of the Ethics Committee of the Far Eastern Federal University. The provisions of the Helsinki Declaration (2000, 2013) were taken into account and taken into account. Biopsies of the oral mucosa were obtained in accordance with the order of the Ministry of Health of the Russian Federation dated 04.29.94 N 82 «On the procedure for conducting pathological autopsies» and in accordance with the nomenclature of clinical and laboratory tests of the Ministry of Health of the Russian Federation (order of February 21, 2000, No. 64). Clinical material for the study was obtained at the Kolot Medical Center (Vladivostok) during 2019-2020. The biological material of 10 human embryos and fetuses, the age of which was determined according to ultrasound data and the Haas rule, was studied. Staining of biopsy sections was performed according to the classical protocol for the method using hematoxylin and eosin.
Results of own research and their discussion
The epithelium of the vestibule of the oral cavity originates from the cutaneous ectoderm, and the epithelium lining the oral cavity itself is of endodermal origin. The mucous membrane of the oral cavity is divided into lining, or covering (the mucous membrane of the lips, cheeks), chewing (the mucous membrane of the hard palate and gums) and specialized, which covers the upper and lateral surfaces of the tongue. In turn, the epithelium of the lining mucosa is non-keratinizing, the epithelium of the lining mucous membrane is non-keratinizing, the epithelium of the masticatory mucosa is multilayered flat keratinizing, and the epithelium of the specialized membrane of the tongue forms the epithelial-connective tissue papillae. The development of these parts of the mucous membrane goes with certain differences, primarily related to the epithelium [3].
It was revealed that at the 3rd week the wall of the oral fossa of the embryo is covered with a single-layer epithelium. The floor of the oral fossa is covered with a two-layer low-prismatic epithelium, and the roof is covered with ciliated epithelium (Fig. 1-4).
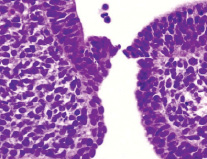
Fig. 1. Oral fossa of a human embryo of 3 weeks. Staining with hematoxylin and eosin. Microphoto. Magnification: х200
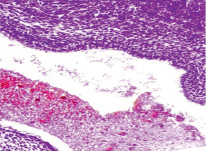
Fig. 2. Oral fossa of a human embryo of 4 weeks. Staining with hematoxylin and eosin. Microphoto. Magnification: х100
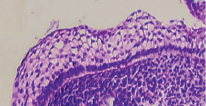
Fig. 3. The oral cavity of a human embryo of 5 weeks. Staining with hematoxylin and eosin. Microphoto. Magnification. х400
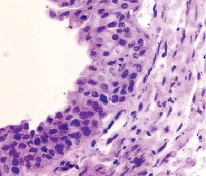
Fig. 4. The oral cavity of a human embryo of 5 weeks. Staining with hematoxylin and eosin. Microphoto. Magnification. x200
The surface of the oral mucosa is represented by stratified squamous epithelium. In the area of the tooth neck, the submerging ectodermal epithelium forms an enamel cap. Behind the neck of the tooth, a two-layer epithelium is revealed. The first is a bright basophilic basal layer, above which squamous epithelium with apically located nuclei is identified. The cytoplasm can be weakly colored or not colored at all. Nuclear-cytoplasmic ratios are high [4].
The mucous membrane is covered with stratified non-keratinized epithelium, under which the basement membrane is identified. In the lamina propria, blood vessels are revealed, the lumen of which is filled with erythrocytes, and numerous fibroblasts are revealed in the intercellular substance with a predominant content of the amorphous substance [5].
We found an increase in the length of the connective tissue papillae protruding into the epithelial layer, a change in the nuclear-cytoplasmic relationship towards their decrease. Differentiation of epithelial cells and their specialization correspond to a gradual increase in the barrier function of the integumentary epithelium of the oral mucosa. The regenerative potential gradually decreases, the structure of the connective tissue is characterized by the appearance of collagen fibers adjacent to the epithelial layer [6].
In the composition of the epithelium of the oral mucosa, cells appear, the cytoplasm of which is vacuolated, there are sufficiently large vacuoles that displace the nucleus to the cytoplasmic membrane. At the border of the prickly and basal layers, single Langerhans cells are located, mainly identified among the epithelium of the mucous membrane, which suggests and indirectly indicates the presence of antigenic presentation in the oral mucosa for restructuring at the stages of maturation. In the lamina propria of the oral mucosa, a leukocyte reaction can be observed, where monocytes, neutrophils and lymphocytes are identified, which are involved in the regulation of physiological regeneration.
It should be noted that the formation of keratinizing and non-keratinizing epithelium of the oral mucosa is determined by differences in the expression of cytokeratins in epithelial cells, and the differences begin to appear from 10-12 weeks of embryogenesis. At the same time, in the mucous membrane of the chewing type, the epithelium forms scallops protruding into its own lamina, as a result of which the border of the epithelium and connective tissue becomes wavy. As a result of proliferation and further differentiation of cells, the epithelial layer is gradually subdivided into layers (basal, prickly, granular and horny). Initially, before the eruption of teeth, keratinization in the epithelium is carried out by parakeratosis and only after their appearance – by orthokeratosis [7].
The source of development of the lamina propria of the mucous membrane is ectomesenchyme. First, the formation of an amorphous substance occurs, after which the process of fiber synthesis begins: at 6-8 weeks of embryonic development, reticular fibers are formed, and collagen fibers appear already at 8-12 weeks. Elastic fibers are formed at 17-20 weeks of development. The differences between the proper laminae of the chewing and lining mucosa are in the greater number of fibers in the mucous membrane of the chewing type. With the development of the latter in the mesenchyme, starting from 6-8 weeks of embryogenesis, clusters of mesenchymal cells appear, which turn into fibroblasts, intensively synthesizing fibers.
Physiological regeneration and maturation of the oral mucosa in human ontogenesis are associated with the constant proliferative activity of the structures of the oral mucosa. They are under the control and regulation of the effector immunocompetent cells of the oral mucosa [8].
Conclusion
Knowledge of the development of the oral mucosa in human ontogeny are necessary to create an innovative platform for cellular technologies in medicine. This would help to exclude the common pathology of the formation of lateral clefts and underdevelopment, leading to non-union of the middle part of the upper lip with its outer part, which is a consequence of the appearance of the cleft lip, or the formation of a gap between the middle and lateral parts of the palate, which forms the cleft palate.
Scientific adviser – Doctor of Medical Sciences, Professor G.V. Reva.

