The development of the software-hardware tools and technologies aimed at building a model of the heart in order to increase diagnostic efficiency is relevant in modern medicine. This is especially true in the direction of predicting the evolvement of pathologies [1]. It is known that for all areas of medical research, both applied and fundamental, modeling of the hemodynamic, physicomechanical and electrophysical characteristics of biological objects is integral part of these studies [2-3]. Currently, a sufficiently large number of models of the heart and cardiovascular system (HCS) have been developed. In medical practice, due to the high level of visualization of the studied structures, as a rule, geometric and physical models of HCS are used [4, 5]. However, due to the large number of possible states caused by both morphological and functional features, such models do not allow to take into account the extensive experimental material accumulated to date. Models that allow to describe the factors and parameters that are significant for the original object are more interesting. Such models of biological objects suggest the presence of three main subclasses, namely hemodynamic, kinetic, and electrical mathematical models. However, the classical mathematical models that describe the processes occurring in the HCS have limited practical application. The theoretical background is based on the postulate of myocardial homogeneity, and is limited to the construction of simplified models that do not take into account the physiological and pathophysiological influence of the phenomenon of myocardial topology. At the same time, a theoretical description of the HCS was set forth in [5, 6] and the problem of mathematical modeling of the HCS was formulated, based on the recent discovery of scientists [4] which showed that the heart myocardium has the Mobius topology. It is argued that the construction of a mathematical model that reproduces the main functions of the HCS, taking into account such anatomy of the myocardium, provides hemodynamic, physical and mechanical and electrophysical characteristics of the HCS, physiologically real and close to the results of clinical observations.
At the same time, it should be noted that currently there is a significant progress in the technical characteristics and functionality of the ECG analyzers. They allow you to visualize informative components related to the field of chaotic dynamics, in particular low-amplitude measurements of the ECG signal in consecutive heartbeats. For example, there are known methods for monitoring myocardial electrical instability, for example, the MTWA method and a number of others, as well as foreign-made devices (CH-2000 from Cambridge Heart, CardioDM 06 from Heart View) and Russian productions (“KardioVizor-06c” ). Their work is based on the registration and analysis of microvolt alternations, which have the form of a random process, and allows us to assess the propensity of the myocardium to develop certain pathologies in the analyzed lead based on the analysis of the dispersion of amplitudes of ECG signals. At the same time, the measured ECG amplitudes of micro-oscillations of electrical voltages in the range of 3 ... 20 μV are comparable in level with the noise signal of the surface ECG, which makes it difficult to analyze the severity of pathological changes in the myocardium. In addition, the recorded fluctuations of electrical signals can be significantly distorted by the heterogeneous nature of the biological tissue surrounding the heart, which greatly complicates the accuracy of the analysis of the amplitude of the recorded electrical signal [7]. Due to these shortcomings, registration of electrical signals is not very suitable for early detection of pathological changes in the myocardium, despite its high sensitivity to electrical instability of the myocardium.
The aim of the study is to analyze the currently existing models of the heart and cardiovascular system, established during the formation of existing systems and methods for diagnosing pathologies based on the postulate of myocardial uniformity.
Hemodynamic model is one of the most famous models of HCS disease at present. [8]. Consider the features of modeling C in the framework of the concept of “myocardium new topology”. It is known [9] that the mathematical description of the flow of blood is based on the use of a system of equations, in which the formal record of a general form on each edge of the graph is:
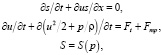 (1)
(1)
where S is the cross-sectional area of a blood vessel; u – linear blood velocity; р – blood pressure; t – time; x – ength along the axis of each vessel; ρ – blood density ρ = const; Ft – external force for example gravity; 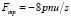 – force of viscous friction of blood flow against vessel walls; n – blood viscosity coefficient.
– force of viscous friction of blood flow against vessel walls; n – blood viscosity coefficient.
From the system of hemodynamic equations (1), consisting of nonlinear differential and algebraic equations, it is clear that the most convenient way to solve it is the numerical method. It is convenient to use software packages of mathematical modeling of objects for the implementation of numerical methods for solving systems of differential equations, such as hardware packages for computing systems like ANSIS, NASTRAN / PATRAN, ELCUT, etc. It should be noted that the listed hardware packages also allow to calculate, for example, non-Newtonian biological fluids (such as blood), to take into account the anisotropic nature of the medium (in particular, the physicomechanical properties of the myocardium), the characteristics of the electromagnetic conducting media to account for the electrical properties of the myocardium. The use of ANSIS, NASTRAN / PATRAN, ELCUT hardware packages makes the development and further complication of a theoretical model and system of equations (1) describing hemodynamic processes promising. This primarily relates to ensuring the operation of highly promising medical equipment, for example, a multifunctional ultrasound device for carrying out cardiovascular research, as well as to other medical devices using micro- and nano-electromechanical components. However, it can be seen that the mathematical model (1) does not allow to take into account the change in the kinetic viscosity of the blood during the cardiac cycle, as well as the difference and dissimilarity in the blood flow regimes, including, for example, the absorption effect of the flow of non-Newtonian fluid. Thus, the hemodynamic model currently cannot be used to solve problems associated with optimizing the blood flow regime in the HCS. It is necessary to additionally include in the system of hemodynamic equations the consideration of the hydromechanical properties and features of HCS for the solving optimization problems. In this case, the description of the blood flow regime, for example, based on the ANSIS and NASTRAN / PATRAN programs, will require the creation of a computational complex describing the hemodynamics of the HCS in the framework of the “myocardium of the new topology” concept in the form of a 3D finite-differential grid of the heart bloodstream. It should be noted that the creation of a hemodynamic model of the HCS with a program is possible on the basis of information obtained with the help of modern ultrasound diagnostic complexes. This will improve the mechanism of operation of electronic sensors, and other components of diagnostic equipment, which suggests that the model created will change the fundamental concepts in this area. Another well-known and no less common classical theoretical model describing the activities of the HCS is the kinetic model [10, 11], whose mathematical apparatus regards the heart as a kind of uniform object that creates pressure and reports blood kinetic energy [12]. Consider the features of the theoretical basis of the kinetic model. It is known that the main parameter of modeling in such a physicomechanical model is heart rate. Then the work of any of the ventricles of the heart in the interval of one contraction can be determined from the form:
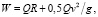 (2)
(2)
where Q is discharge of blood from the ventricle; R – blood flow resistance; g – acceleration of gravity.
It can be seen that in the kinetic model (2) the work of the heart for one heartbeat in general depends not only on the transfer of a certain amount of blood in the arteries, but also is associated with the formation of elastic tension in the cardiac muscle. Within the framework of the “myocardium of the new topology” concept, this makes it relevant to develop a functional model of the heart. It should describe the physicomechanical processes for multi-frequency broadband electronic sensors when forming a beam for reception / transmission in multifunctional diagnostic medical devices for conducting ultrasound studies of the HCS.
As was shown above, one of the new directions in modeling processes and building a simulation model of the work of the HCS is based on the representation of the myocardium as a figure with the Mobius topology [13]. The consideration of the critical quality properties and features of the myocardium of the heart in the description of the work of the HCS allows us to consider the myocardium as a combination of a combination of a set of magnetic domains. This, in turn, makes it possible to link the biomechanics of heart muscle contractions and the hemodynamics of blood flow in elastic vessels in close interaction with electrically conductive processes that regulate HCS in general:
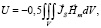 (3)
(3)
where V is a magnet volume; JS. – magnetization; Hm – magnetic field strength.
The ideas about the structure of the myocardium in the form of a sheet rolled up in accordance with the Moebius topology raise the question of further studying the effect of coherence and orientation of the entire circulatory system and its conjugation with the heart, since the Mobius transformation is a composition of a finite number of inversions relative to spheres in Euclidean space 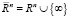 . Moreover, the set of all Moebius transformations of space
. Moreover, the set of all Moebius transformations of space 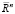 is finite-dimensional, and the subgroup composed of its orientation-preserving mappings is isomorphic [14]. The isomorphism of a subgroup made up of mappings is of particular importance in mathematical modeling in the framework of the transformer model of the heart. According to this model, the emission of the magnetic field created by the myocardium of the heart is described in the approximation that the myocardium of the heart is a magnetic core, which is formed by materials with an oriented domain structure. Indeed, it can be seen from (3) that for a space filled with magnetic material with magnetization JS, the magnetostatic interaction of separate volume elements inside a magnetized body leads to the presence of its own magnetostatic energy of this body, and the magnitude of the stray field Hm leads to the formation of spontaneous magnetization regions, i.e. domain structure. This allows us to consider the myocardium of the heart as a magnetic domain structure, namely, a set of regions in the magnetic subsystem of magnetic materials that associate the microscopic magnetic characteristics with their macroscopic properties. Thus, we accept during the mathematical description of the processes of formation in the myocardium of the magnetic field that the magnetization and the magnetization reversal is determined by the properties of the domain structure.
is finite-dimensional, and the subgroup composed of its orientation-preserving mappings is isomorphic [14]. The isomorphism of a subgroup made up of mappings is of particular importance in mathematical modeling in the framework of the transformer model of the heart. According to this model, the emission of the magnetic field created by the myocardium of the heart is described in the approximation that the myocardium of the heart is a magnetic core, which is formed by materials with an oriented domain structure. Indeed, it can be seen from (3) that for a space filled with magnetic material with magnetization JS, the magnetostatic interaction of separate volume elements inside a magnetized body leads to the presence of its own magnetostatic energy of this body, and the magnitude of the stray field Hm leads to the formation of spontaneous magnetization regions, i.e. domain structure. This allows us to consider the myocardium of the heart as a magnetic domain structure, namely, a set of regions in the magnetic subsystem of magnetic materials that associate the microscopic magnetic characteristics with their macroscopic properties. Thus, we accept during the mathematical description of the processes of formation in the myocardium of the magnetic field that the magnetization and the magnetization reversal is determined by the properties of the domain structure.
When transforming the topology of the magnetic circuit, a change in the magnetic field strength is observed. In particular, its change from a toroidal form to a form in the form of a Mobius loop is observed. This is caused by the effect of a change in domain orientation. It allows us to solve the problem of “visualization” of the internal structure of the myocardium of the heart according to the results of the analysis of the magnetic field strength. The amplitude values of the magnetic field strength differ for normal (twisted myocardium) and pathologies by up to 2 times. The reliability of the assumptions made (3) is qualitatively confirmed by the results of a physical experiment conducted in the form of clinical observations [15].
A new approach to modeling the HCS man, allows you to link together electro-biomechanical processes. This allows early diagnosis of the evolvement of pathologies based on the assessment of changes in the characteristics of the functioning of the HCS. In this case, the CCC model can be represented as a 3D finite element model describing the physicomechanical processes occurring in the heart. It is based on the study and proof of the results of the following hypotheses: the spiral mode of oscillations is a significant characteristic of a healthy heart (this is the basis for early diagnosis of pathologies); the energy of a healthy heart is 4 times less than the energy of a sick heart (the configuration of a cylindrical ring is the basis for predicting the myocardial fiber deceleration); muscle stimulation of the myocardium spreads in the form of a solitary “compression-tension” wave.
The analysis of currently existing models of the heart and cardiovascular system, assuming homogeneity of the myocardium, showed interest in the revision and further development of the classical theories used to describe the electrical, bio-, mechanical processes in the cardiovascular system. Taking into account the fact that the physiological and pathophysiological significance of the phenomenon of myocardial topology has not been fully studied yet, there is a high need for a system-synergetic approach to a fundamental rethinking and revision of the existing mathematical models describing the work of the heart and cardiovascular system in terms of the new, critically important qualitative properties and features of the myocardium of the heart.
Conclusion
Based on a practical approach to the study of electrical and mechanical functions in comparing the homogeneous and heterogeneous myocardium of the heart, the paper analyzes the existing mathematical models describing the work of the HCS. A distinctive feature of this approach is the possibility of identifying the need to take into account the physiological and pathophysiological significance of myocardial heterogeneity. The system-synergetic approach to modeling the HCS takes into account the synchronization between the various processes that form the electro-biomechanics of the functioning of the HCS, and this will make it possible to better match the local and non-local characteristics to the results of physiological experiments and clinical observations. This will contribute to the growth of efficiency and accelerate the adaptation of new modeling tools in clinical applications both for the diagnosis of cardiac activity, and for the development of electromechanical stimulants of cardiovascular system and target simulators for cardiac surgeons. Consequently, reliability will increase and the probability of effective surgical operations on the cardiovascular system will increase.

