Calcium chalcogenides and the triple phases obtained on their basis are related to promising substances for the development of luminescent and photoelectrical materials. Solid solutions of calcium and gallium chalcogenides exhibit luminescent properties of practical interest [3-8]. In the interaction of alkaline earth metal chalcogenides with gallium chalcogenides, threecomponent compounds and solid solutions are formed, which must preserve the properties of the initial binary compounds, and at the same time have more pronounced complex properties.
The synthesis of the system of the GaTe-CaTe alloys consists of GaTe and CaTe components, which are synthesized at around 850-900 °C in an quartz ampoule that has 0.13333p air sucked from it. The process of the synthesis is done in two stages: In the first stage the mixture is heated up to 900 °C and maintained the same temperature for twenty four hours. In the second stage temperature is raised from 900 to 1000 °C and synthesized for two hours [1, 2]. The alloys which are rich of GaTe compound are tending to show dark brown color and the alloys which are rich of CaTe compound tend to show dark ash color. The alloys which are rich of GaTe compound are resistant to air and water. They tend to well dissolve in mineral solvents. On the other hand the alloys which are rich of CaTe compound are resistant to air while they can absorb moisture from air and changing their appearance in process.
To homogenize the system of GaTe-CaTe alloys is maintained during 500 hours at 650 °C temperature which thermally processes the alloys. Then the system GaTe-CaTe alloys is analyzed by physical and chemical methods of analysis and phase diagrams are plotted. The system GaTe-CaTe is the quasi-binary crop of Ca-Ga-Te three way systems, and it is characterized by using peristaltic compound and limited dilution of components. In this system components make a three way compound CaGaTe2 in ratio of 1:1 and melts incongruently at 860 °C.
M + CaTe ↔ CaGaTe2.
The GaTe-CaTe system was studied by differential thermal analysis (DTA), X-ray diffraction (XRD), micro structural analysis, density measurements, and micro hardness tests. DTA curves were obtained at a heating rate of 8 °C/min on a NTR-73 low-regency thermal analyzer. Diffraction patterns were collected on a D2PHASER X-ray diffract meter with CuKα radiation. Micro hardness values were determined on a Thixomet Smart Drive micro hardness tester at indentation loads chosen after micro hardness measurements for each of the phases present. The microstructure of the alloys was investigated by MIM-8 microscope. Glory sections of the alloys were etched with a 1:2 mixture of concentrated HNO3 and H2O2. The DTA, microstructural analysis, XRD, micro hardness and density data were used to map out the T-X phase diagram of the GaTe-CaTe system (Fig. 1).
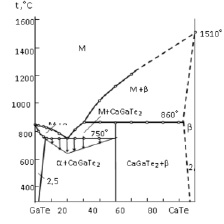
Fig. 1. Phase diagram of the GaTe-CaTe sect
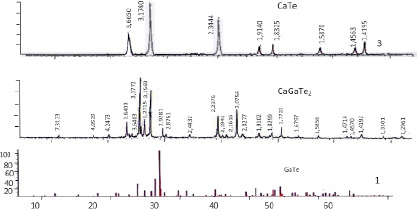
Fig. 2. X-ray diffraction patterns for GaTe-CaTe alloys containing: 1 – GaTe, 2 – CaGaTe2, 3 – CaTe
Based on gallium monotellurid dilution of CaTe is 2.5 mol %.The solid percentage of CaTe olution is not detected, the solid part of the solution GaTe is 2.0 moll %.
Microstructure analysis revealed that, GaTe based dilution at room temperature is 2.5 %mol CaTe, at 750 °C approximately 9 moll % CaTe.
Based on CaTe compound solid matter cannot be detected that is why solid matter is considered approximately as 2,0 moll % and its border is represented as jerky lines. CaGaTe2 compound makes eutectics with gallium monotellurid and its coordinates is 20 %moll CaTe its melting point is 750 °C. Tamman triangle should be assembled in order to find the exact location of eutectics. To find the existence of CaGaTe2 compound x-ray phase analysis was made.
According to the results of x-ray phase analysis calculated distance between planes and diffraction maximum’s intensity are being compared to CaGaTe2 compound and primary components. (Fig. 2) As seen from figure 2 in the difractogram of CaGaTe2 compound determined diffraction maximums differ from primary components based on the difference between planes and intensity.
This proves that, inside of GaTe-CaTe system new three way compound CaGaTe2 is produced. CaGaTe2 compound’s x-ray results are shown in table 1.
Table 1
The interlunar spacing?s (dα?), hkl indices, and relative peakintensities in the XRD pattern of the CaGaTe2
|
№ |
I |
2θ |
d exp. |
d count. |
1/d2 exp. |
1/d2 count. |
hkl |
|
1 |
3,5 |
12,094 |
7,3123 |
7,3123 |
0,0187 |
0,0187 |
100 |
|
2 |
3,6 |
18,266 |
4,8529 |
4,9752 |
0,0425 |
0,0404 |
101 |
|
3 |
4,1 |
20,898 |
4,2473 |
4,1416 |
0,0554 |
0,0583 |
111 |
|
4 |
26,2 |
24,432 |
3,6403 |
3,6564 |
0,0755 |
0,0748 |
200 |
|
6 |
100 |
26,369 |
3,3772 |
3,3748 |
0,0877 |
0,0878 |
002 |
|
6 |
29,7 |
27,237 |
3,2715 |
3,2686 |
0,0934 |
0,0936 |
212 |
|
7 |
78,5 |
28,248 |
3,1568 |
3,0642 |
0,1035 |
0,1065 |
102 |
|
8 |
15,5 |
30,505 |
2,9280 |
2,9424 |
0,1166 |
0,1155 |
211 |
|
9 |
5,7 |
31,070 |
2,8761 |
2,8375 |
0,1209 |
0,1242 |
112 |
|
10 |
1,7 |
36,755 |
2,4432 |
2,4368 |
0,1675 |
0,1684 |
300 |
|
11 |
37,7 |
40,274 |
2,2375 |
2,2502 |
0,1997 |
0,1975 |
003 |
|
12 |
2,5 |
41,108 |
2,1940 |
2,1874 |
0,2077 |
0,2090 |
311 |
|
13 |
3,7 |
41,752 |
2,1616 |
2,1507 |
0,2140 |
0,2162 |
103 |
|
14 |
48,6 |
43,571 |
2,0756 |
2,0677 |
0,2321 |
0,2339 |
113 |
|
15 |
5,3 |
44,654 |
2,0277 |
2,0274 |
0,2432 |
0,2433 |
320 |
|
16 |
9,0 |
47,564 |
1,9102 |
1,9163 |
0,2740 |
0,2723 |
203 |
|
17 |
9,2 |
49,790 |
1,8299 |
1,8276 |
0,2986 |
0,2994 |
400 |
|
18 |
16,3 |
51,528 |
1,7722 |
1,7730 |
0,3184 |
0,3181 |
410 |
|
19 |
2,6 |
54,597 |
1,6797 |
1,6874 |
0,3544 |
0,3512 |
004 |
|
20 |
4,1 |
58,131 |
1,5856 |
1,5887 |
0,3977 |
0,3962 |
421 |
|
21 |
1,6 |
63,136 |
1,4714 |
1,4711 |
0,4619 |
0,4621 |
422 |
|
22 |
1,8 |
63,835 |
1,4570 |
1,4621 |
0,4711 |
0,4678 |
500 |
|
23 |
6,1 |
65,747 |
1,4191 |
1,4186 |
0,4966 |
0,4969 |
403 |
|
24 |
3,5 |
69,575 |
1,3501 |
1,3500 |
0,5486 |
0,5487 |
005 |
|
25 |
3,3 |
72,929 |
1,2961 |
1,2929 |
0,5953 |
0,5988 |
440 |
Table 2
Results of DTA, micro hardness and density data for alloys GaTe-CaTe system
|
composition, mol % |
thermal effects of heating, oC |
density, q/sm3 |
micro hardness, MPa |
||||
|
Ga2Te3 |
CaTe |
α |
CaGa4Te7 |
CaGa2Te4 |
CaTe |
||
|
P = 0,20 N |
P = 0,15 N |
||||||
|
100 |
0,0 |
812 |
5,57 |
2370 |
- |
- |
- |
|
98 |
2,0 |
720,790 |
5,57 |
2430 |
- |
- |
- |
|
95 |
5,0 |
650,770 |
5,58 |
2450 |
- |
- |
- |
|
93 |
7,0 |
630,750 |
5,60 |
2480 |
- |
- |
|
|
90 |
10 |
580,725 |
5,61 |
2500 |
- |
- |
- |
|
85 |
15 |
580,685 |
5,57 |
- |
- |
- |
- |
|
80 |
20 |
580 |
5,46 |
Evtek. |
Evtek. |
- |
- |
|
75 |
30 |
580,610 |
5,40 |
- |
2150 |
- |
- |
|
70 |
30 |
580,680 |
5,37 |
- |
2150 |
2200 |
- |
|
66,6 |
33,3 |
680, 760 |
5,35 |
- |
2150 |
2200 |
- |
|
60 |
40 |
680, 800 |
5,20 |
- |
2150 |
2200 |
- |
|
55 |
45 |
690,850 |
5,06 |
- |
- |
2200 |
- |
|
50 |
50 |
870 |
4,90 |
- |
- |
2200 |
- |
|
45 |
55 |
735,830 |
4,80 |
- |
- |
- |
- |
|
40 |
60 |
730 |
4,76 |
- |
- |
Evtek. |
Evtek |
|
30 |
30 |
730,960 |
4,70 |
- |
- |
- |
1800 |
|
20 |
80 |
730,1150 |
4,66 |
- |
- |
- |
1800 |
|
10 |
90 |
730,1335 |
4,50 |
- |
- |
- |
1800 |
|
0,0 |
100 |
1510 |
4,35 |
- |
- |
- |
1800 |
It is defined that, CaGaTe2 is crystallized in tetragonal syngony and its cell properties are a = 7.31, c = 6.75; Z = 3; ppikn = 4.82 g/cm3; pprent = 4.92 g/cm3.
During the measurement of micro strength in the system, it tends to show three different values. From six hundred to seven hundred MPa values represent the alpha solid solution which is obtained from Gate compound, (1500-1550) MPa values represent the strength of CaGaTe2 compound, while (1800-1860) values of micro strength represent beta solid solution which is made from CaTe compound. GaTe-CaTe system’s liquids is made from alpha solid solution’s slope when the liquid is released in the first crystallization process, and beta solid solution’s slope in monotype equilibrium. The mixture of alpha – phase and CaGaTe2 compounds crystallize in double eutectics point, its composition is 20 moll % CaTe at 750 °C. In between 2.5-50 moll % CaTe concentration interval downwards the solidus line two phase alloys (α+ CaGaTe2) and at 50-98 moll % CaTe concentration interval two phase alloys (b+ CaGaTe2) precipitate.
Thus, The GaTe-CaTe system is analyzed and its phase diagram is sketched. It is defined that phase diagram of the system is quasi-binary and it is characterized with the production of CaGaTe2. CaGaTe2 melts incongruently at 860 °C. According to the primary components it is found that solid solution exists in restricted space. The microstructure analysis revealed that at room temperature 2.5 moll % CaTe dissolves in GaTe while, based on CaTe chemical compound the area of solid solution is 2.0 moll % GaTe. According to the X-ray analysis the crystal type of CaGATe2 compound was found and its cage properties are calculated.
103/T,K Single crystals of the CaGaTe2 compound were first grown by chemical vapour transport, with I2 as a transport agent, but such crystals were unsuitable for physical property measurements, so we prepared single crystals of the CaGaTe2 compound by the Bridgman-Stock Barger method. These single crystals were used in electrical measurements. Fig. 3 shows the Arrhenius plot of electrical conductivity for the CaGaTe2 compound. The data demonstrates intrinsic conduction with activation energy ΔE = 1,12eV.
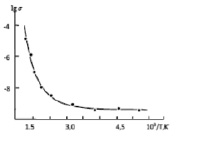
Fig. 3. Arrhenius plot of electrical conductivity for CaGaTe2
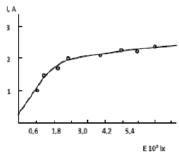
Fig. 4 Current-light curve of CaGaTe2
Fig. 4 presents a room-temperature current light curve of a CaGaTe2 single crystal at an applied voltage of 100 V. at low light intensity, there is only one type of recombination level between quasi-Fermi levels, which has identical capture cross sections for electrons and holes. Another type of level is too deep to be regarded as a recombination level.
The triple compounds CaGaSe2, CaGaTe2, and solid solutions based on them exhibit intensely luminescent properties. When 0.01-0.05 mole % of the rare-earth elements (Eu, Ce, Gd, Th, Sm, Nd) are added to the CaGaSe2, CaGaTe2 alloy and solid solutions based on them, the luminescence efficiency increases by a factor of 5-3. The spectra of photoluminescent crystals CaGaSe2, CaGaTe2 taken during the excitation of the crystal by laser radiation of medium power of excitation of the crystal by laser radiation of average power 0.8 Bm.
The long-wave edge of the photoluminescence spectrum of the CaGaTe2 crystal is formed by annihilation of excitons. The energy of the exciton peak is 1.34 eV. The radiation intensity of exciton radiative recombination varies quadratically with the intensity of the exciting laser radiation. Taking into account that the photon energy of laser radiation is 1.167 eV, then it can be assumed that the excitation of nonequilibrium electrons occurs due to two absorption photons. The observed peaks in the photoluminescence spectra in the high-energy region attest to the reliability of this assumption.

