Background. Pancreatic B-cells contained a large amount of Zn+2ions [1-3] as salivary glands and prostate. In B-cells Zn+2-ions take part in processes of biosynthesis of insulin as at processes of storage by forming of Zn+2-insulin complex [4,5]. It is known that Zn+2-ions in B-cells formed with insulin a deposited form of hormone as Zn+2-insulin complex [4].Proinsulin forms a zinc contain hexamer soon after its synthesis. In addi- tion the Zn+2-ions enhance proinsulin’s solubility and render insulin insoluble. Zinc ions also appear to play an important role in the microcrystalline character of the precipitated insulin granule[5]. Pancreas of rat, rabbit, dog, cat, some fish, human, birds, mice, ham- ster, porcine, hoerst, contain a large amount of Zn+2-ions [6]. Using of electron micro- scopy histochemical method it was showed that that Zn+2-ions are concentrated in B-cells in B-granules only contained deposited form of insulin [7] and destruction of B-cells caused by Dithizon which formed in B-cells toxic complexes with Zn+2-ions, star- ted by destruction of B-granules [8].
There are a few histochemical methods for staining of insulin or zinc-insulin complex in B-cells for to reveal and to estimate insulin content in B-cells:
Aldehyde-fucshine method by Gomori G. Violet granules in cytoplasm of B-cells correspond to deposited form of insulin [10-11]. Intensity of color of cytoplasm of B-cells directly correspond to insulin content in cytoplasm [12-13]. But this method is not high specific for insulin only.
Staining by Dithizon. Preparing of Dithizon solution: 30 mg of Dithizon, (MERCK, Germany) +10 ml. bidistillate+0.2 ml 25 % NH4OH 10 min. mixing on temperature +700 at Celsium. Solution was injected intravenously to Rabbits and to Mice 46-48,6 mg/kg.
Frozen sections of 4 mcm were investigated 5-10 min past injection on dark mic- roscopy. Density of staining was measured using photometer. Insulin content was calcu- lated as parameter K = AB1/AB2 where: AB1-density of staining of intact B-cells; AB1-density of staining of B-cells past action of diabetogenic chemicals (calculated as 1,00).
Immunohistochemical method staining of insulin. Standart kits for insulin (DAKO, Demark) were used for staining sections of pancreas tissue.
Diethylpseudoisocyanine fluorescent method. Schiebler T. and Schiessler S. showed that A chair of oxidized insulin reacted with Diethylpseudoisocyanine chloride with formation of red fluorescent complex which fluoresces in UV light 360-370 nm. We have used modernized by Coalson R.E.method [14-15, 20].
Description of staining procedures. Preparing of staining solution: 0,04 % water solution of Diethylpseudoisocyanine (SERVA,Germany). Staining procedures: 1) depa-raffinization of sections in xylol; 2) alcohol 900,800,700 1 min in each;3)washing in cold water; 4) oxidation 0,5-2 min; oxidation solution: 5 ml of 5 % H2SO4+5 ml 2,5 % solution of KMnO4+30 ml bidistilled water at +280 Celsius; 5) washing in cold water; 6) 5 % solution of oxalic acid – 5 sec; 6) washing in 2 portions of cold water; 7) 0,4 % cold solution of Diethylpseudoisocyanine – 20 min in refrigerator at +40Celsius; 8) washing in cold water 5 min; 9) store in refrigerator 1,5-3h.
Staining of Zinc in B-cells by 8PTSQ (from Institute for High Pure Chemicals, Moskva, Russia). Zn+2-8PTSQ complex radiates intensive green fluorescence under UV-light 360-370 nm length of wave [16-19,21] that was confirmed by spectral analysis [8]. Cytoplasm of B-cells not contained Cadmium. Past long time prolonging testing in Institute of High Pure Chemicals (Moscow) 8PTSQ was proposed as fluorescent reagent for identification of very small amounts of Zn+2 in solutions and tissues. Later by Lasaris Y.A. and coll. 8PTSQ was tested for revealing Zn+2-ions. 8PTSQ is high specific reagent for staining of Zn+2-ions in pancreatic B-cells. Concentration of Zinc-ions in cytoplasm of B-cells is proportional for concentration of insulin.
Victoria Blue 4R method staining of insulin (V4R), Diphenylnaphthylmetane, colour index 42563; MERCK, Germany; FERAK, West Berlin, Germany). It was showed by F. Wohlrab [16] that V4R in aqueous solution interacted with oxidized A-chair of insulin that is accompanied by blue staining of cytoplasm of В-cells proportionally to the amount of insulin [18].
Insulin content was calculated as parameter K = AB1/AB2 [25] where: AB1-density of staining of intact B-cells; AB1-density of staining of B-cells past action of diabetogenic chemicals (calculated as 1,00).
Aim of work: 1) staining of insulin in B-cells using of histochemical Victoria Blue 4R method; 2) to compare with results obtained by using other methods of staining of insulin and Zinc.
Methods. Staining reagents: Aldehyde-fucshine (MERCK, Germany), Diethylpseudo- isocyanine (SERVA, Germany), Dithizon (MERCK, Germany), Dimethylnaphtylmetan (Victoria 4R) (FERAK,West Berlin), immunohistochemistry (standard kits from DAKO, Denmark), 8-para (toluenesulphonylamino)quinolin -8PTSQ (from Institute for High Pure Chemicals, Moskva, Russia).
Animals. 11 Rabbits 2250-2720g. Group 1.Intact animals (3). Group 2. Experimental diabetes induced by injection of 48,9-52,2 mg/kg of Dithizon (Diphenylthiocarbazon, SERVA,Germany).4 animals were killed 10 min. after injection of Dithizon and 4 animals – 8-9 days after injection.
Frosen sections of pancreas of animals were investigated using dark microscopy. Blood glucose level measuring – in animals of 2a and 2b groups before injection of Dithizon and 1,3,6 and 9 days after injection. Aldehyde-fucshine (MERCK, Germany) method [10-13] and Diethylpseudoisocyanine methods were used for analysis state of histostructure of pancreas tissue and of deposited insulin content in B-cells [14-15] as a specific fluorescent 8PTSQ and Dithizon methods for staining of complex “Zn-DZ” and of free ions of Zn in B-cells [8,9,16-21]. 8TSQ formed fluorescent green complexes with Zn+2-ions visible using fluorescent microscopy and Dithizone formed red DZ-Zn+2-ions complex visible using dark microscopy. Maximum of absorbance of Zn+2-DZ complex on spectrum of absorbance correspond for 530 nm [4]. The Victoria 4R stai- ning technology was used for staining of insulin [22-24]. Insulin content was calculated in relative units (r.u.) as parameter K = IF1/IF2 where: IF1-intensity of fluorescence of intact B-cells (B-cells/exocrine tissue); IF2-intensity of fluorescence of B-cells after action of diabetogenic chemicals (B-cells/exocrine tissue). Histofluorimetric complex was used [25] for to investigate intensity of fluorescence and density of staining of insulin in B-cells.
Preparing of solutions
Preparing of Ditizon solution: 400 mg+30 ml of bidistillate+0,2 ml of 25 % ammonium solution; mixing 10 min ot water bath at +700C, filtration. Frozen sections of Rabbit’s pancreas 4 mcm were investigated 10 min after injection using of dark-condensor microscopy. Intensity of staining was measured by photometer. 2nd part of pancreas tissue was fixed in Ethanol 70 % contains dissolved H2S; paraffin sections of tissue were stained by 0,4 % acetone solution of 8PTSQ [6, 12, 13] and were investigated on fluorescent microscope. Pancreas tissue was fixed in Bouin 24h.


1 2 3
4 5 6
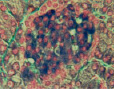
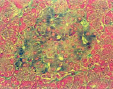
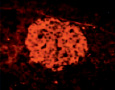
7 8 9

10
Fig. 1. State of histostructure and insulin content in B-cells of intact animals and animals with diabetes caused by Dithizon (histological materials, staining and microphotos by Meyramov G.G.and co-authors)
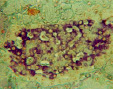
1.1. Intact rabbit. Pancreas. Staining by Dithizon. Large amount of red granules of Zinc-Dithizon complex in B-cells. Histostructure of B-cells without changes; x280;
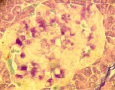
1.2. Diabetes. Pancreas. Staining by Dithizon. Absence of Zinc in B-cells; x280;
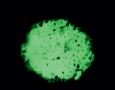
1.3. Intact rabbit. Pancreas. Fluorescent positive Zinc reaction with 8PTSQ in B-cells (intensive green fluorescence of cytoplasm of B-cells. Histostructure of B-cells without changes; x140;
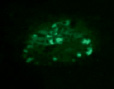
1.4. Diabetes. Pancreas. Fluorescent negative Zinc reaction with 8PTSQ in B-cells (absence of fluorescence of cytoplasm of B-cells). Staining by 8PTSQ; x140;
1.5. Intact rabbit. Pancreas. Aldehyde-fucshine staining. Histostructure and insulin content (violet color) in B-cells without changes; x280;
1.6. Diabetes caused by Dithizon Pancreas.Aldehyde-fucshine staining. Destruction of B-cells and marked decreasing of insulin content in B-cells; x280;
1.7. Intact rabbit. Pancreas. Staining by Victoria 4R. Positive reaction for insulin in B-cells (intensive blue color of cytoplasm of B-cells). Histostructure and insulin content in B-cells without changes; x280;
1.8. Diabetes. Pancreas. Staining by Victoria 4R. Negative reaction for insulin in B-cells. Destruction of B-cells and marked decreasing of insulin content; x280
1.9. Intact rabbit. Pancreas. Staining by Diethylpseudoisocycnine. Positive reaction for insulin in B-cells intensive red fluorescence). Histostructure and insulin content in B-cells without changes; x140;
1.10. Diabetes. Pancreas. Staining by Diethylpseudoisocycnine. Negative reaction for insulin in B-cells (low fluorescence); x140
Research results and discussion
Group 1. Pancreas tissue of intact animals
Staining by Dithizon: a large amount of zinc-insulin complex (red granules) in B-cells (Fig. 1.1).
Aldehyde-fuchsine staining: histostructure and insulin content in B-cells (violet color) without changes, (fig.1.5; Table); insulin content in B-cells: K = 1,80 ± 0,06.
Victoria 4R staining: : histostructure and insulin content in B-cells without changes (Fig.1.7.;Table); insulin content in B-cells: K = 1,62 ± 0,05.
Immunohistochemistry: histostructure and insulin content in B-cells without changes (Table); insulin content in B-cells: K = 1,74 ± 0,04.
Diethylpseudoisocyanine staining: histostructure and insulin content in B-cells without changes (Fig.1.9;Table); insulin content in B-cells: K = 1,72 ± 0,04.
Fluorescent staining of Zn+2-ions: a large amount of Zn-ions in B-cells: intensive green fluorescence of B-cells (Fig. 1.3, Table); Zn-ions content in B-cells: K = 1,75 ± 0,03.
Group 2. Pancreas tissue after action of Dithizon
Staining by Dithizon: a low amount of zinc-insulin complex (red granules) in B-cells (Fig.1.2.).
Aldehyde-fuchsine staining: destruction and death of majority of B-cells, marked de- creasing of insulin content in B-cells (fig.1.6; Table); insulin content in B-cells: K = 1,12 ± 0,03
Immunohistochemistry: destruction and death of B-cells; marked decreasing of insulin content (Table); insulin content in B-cells: K = 1,03 ± 0,02.
Diethylpseudoisocyanine staining: marked decreasing of insulin content (Fig.1.10; Table); insulin content in B-cells: K = 1,11 ± 0,04.
Victoria 4R staining: destruction and death of majority of B-cells, marked de- creasing of insulin content in B-cells (Fig.1.8;Table); insulin content in B-cells: K = 1,08 ± 0,09
Fluorescent staining of Zn+2-ions, DZ: absence of Zn+2-ions in cytoplasm of B-cells (Fig.1.4; Table); Zn-ions content in B-cells: K = 1,04 ± 0,01
Results of comparative analysis of histochemical identification of insulin in pancreatic B-cells using of various methods shown following. All methods demon- strated some differences of insulin content and state of histostructure of pancreas tissue in animals with diabetes in comparison with intacts. Concerning insulin staining two from five methods – Victoria 4R and Diethylpseudoisocyanine method are belong for high specific methods for staining of A-peptide of molecule of insulin. In the contrary, Aldehyde-fucshine method and staining by Dithizon method are not belong to specific for staining of insulin and zinc-insulin complex only.
Not only insulin but some like hormone substances from adenohypophysis accepted Aldehyde-fucshine color. However, regarding pancreatic islet tissue it is possible to recognize this method as specific for insulin because other hormones in B-cells are not produced. Staining by Dithizon result color revealing of complex zinc-insulin as red granules in B-cells. Thus, it is possible to determine the content of insulin indirectly only.
The advantage of Diethylpseudoisocyanine method determined by high sensitivity in compared with Victoria 4R method. Shortcomings: 1) histologic slides of pancreas tissue are changeable a limited time only -20-30 min – for microscopic investigation; 2) this method is belong for histochemical technics and not suitable for to investigate state of histostructure of pancreas tissue.
Comparative analysis results of measuring of insulin content in B-cells using of various methods (r.u., parameter K)
|
№ |
Method |
Intact animals |
Diabetes induced by Dithizon |
Difference of Indexes |
|
1 2 3 4 |
Pancreas tissue Aldehyde-fucshine Victoria 4R Immunohistochemistry Diethylpseudoisocyanine 8PTSQ (zinc reaction) |
1,86 ± 0,05 1,72 ± 0,06 1,90 ± 0,04 1,92 ± 0,06 2,05 ± 0,07 |
1,12 ± 0,03 p < 0,005 1,08 ± 0,09 p < 0,005 1,03 ± 0,02 p < 0,005 1,11 ± 0,04 p < 0,005 1,04 ± 0,01 p < 0,005 |
0,89 0,63 0,84 0,93 |
Advantages of Victoria 4R method: 1)fixation of color using of paraffin histological sections of pancreas tissue for microscopy within long time and storage of slides for long period; 2) at the same time the method is suitable for the investigation and description of histological changes of pancreas tissue not only for staining of insu- lin. This is two of his important advantages.
Both methods do not belong to difficult methods on technical aspects. Dimethyl- naphtylmetan – a main color reagent for staining process by Victoria 4R -is inexpensive and is produced by many European firms.
This method is used not often, that is why we propose description of staining procedures. Staining procedures:
1) deparaffinization of sections;
2) washing in cold water a few min;
3) oxidation 3-5 min(oxidation solution: 0,3 % KMnO4 50 ml + 0,3 % H2SO4 50 ml; wash slides;
4) place sections in 2-5 % water solution of sodium bisulphate – 1 min; wash slides;
5) 700 alcohol-1 min;
6) staining solution (960 alcohol 100 ml + Victoria Blue 4R – 1g) 15 min – 2h; wash slides;
7) staining on 0,5 % water solution of Phloxine 30-120 sec.; wash slides;
8) 5 % water solution of phosphor wolframic acid 1-2 min; wash slides; in water;
9) staining in 0,5 % water solution of Light Green 1-2 min;
10) dehydratation in 96 % alcohol.
Insulin content was calculated as parameter K = AB1/AB2 where: AB1-density of staining of intact B-cells; AB1-density of staining of B-cells past action of diabetogenic chemicals (calculated as 1,00).

