The specific properties of materials of ultradispersed (nano) open up broad opportunities for creation of new effective catalysts, sensor, semiconductor, superconducting systems, drugs with high biological activity for use in the electronics, alternative energy, ecology, medicine and agriculture [1].
Nanomaterials derived from metal cadmium is poorly understood, mainly devoted to the preparation and study of ZnO nanorods, nanotubes of cadmium chalcogenides. For example, in [2], metallic cadmium nanowire obtained by thermal decomposition of cadmium sulfide powder in argon atmosphere. There is a hydrothermal method for producing cadmium hydroxide nanowires by processing 0.01 molar solution of cadmium dehydrate acetate with 0.015 molar solution of hexamethylentetramine for 16 hours at a temperature of 95 °C with the formation of cadmium hydroxide nanowires, which, when further calcined in the air for three hours at a temperature of 350 °C, turn into cadmium oxide nanowires [3, 4].
The disadvantages of the above described methods for producing nanowires of metal cadmium, cadmium hydroxide is the use of high temperatures for decomposition of cadmium sulfide powder, the need for an inert atmosphere, refrigeration and anti-explosion devices, which complicates the hardware design of the process, requires significant energy and material costs. To obtain cadmium hydroxide, it is necessary to maintain long-term high volumes of aqueous solutions in explosive autoclaves. Indeed, currently, the synthesis of nanomaterials requires the use of expensive and sometimes dangerous tools (laser technology, high-frequency ultrasonic devices, explosive technology, hydrothermal synthesis at high temperatures and pressures), or bulk for the use of liquid solutions “wet” chemistry. All known technologies of obtaining nanostructures of cadmium along with its advantages there are also some certain drawbacks in this regard, the search for promising methods of obtaining is actual.
Recently, very interesting are the regularities of the formation of nanoscale objects on the border of two phases, which open the possibility of creating a fundamentally new generation of nanoustroystv, multi-level architecture which is based on the unique property of nanoparticles spontaneously unite into ordered ensembles, both in the volume of dispersion and on interphase surfaces [5]. There are different approaches to the creation of ordered nanostructures at interfaces. Of the microemulsion type “water in oil” (or reverse micelles) have recently attracted increasing attention as microreactors for synthesis of nanoparticles. As is known, highly dispersed water droplets are also ideal micro-reactors for producing microparticles, and the size of droplets is a natural limiter of the sizes of grown nanoparticles [6].
The properties of microemulsions are largely determined by the size and shape of the particles of the dispersed phase, as well as rheological properties of the interphase adsorption layers formed by surfactants. Since microemulsions have a high mobility and a large surface area between the phases, they can serve as a universal medium for many chemical syntheses, including for the production of solid nanoparticles. It is known that phase interface surfaces often have unique properties. Studies on the boundaries of the phases have already made great strides in a wide variety of areas of expertise.
Experimental part
To obtain nanostructures, we have proposed pulsed plasma in the liquid (PPL) [7] and microemulsion, which is created with the help of a high-speed magnetic stirrer.
Low-voltage pulsed plasma in liquids occurs as a result of an electric breakdown of the interelectrode space at a relatively low power source, insufficient to excite the arc plasma. Unit impulse localized in time and has duration of the order of 10-3-10-5 s. In the zone of influence of a single pulse (10-3-10-4 см3) develop high temperatures (104-105 K) due to the high current density (106-108 A/cm2) and pressure (3-10 Kbar), resulting from the transience of the process. The energy of a single pulse is sufficient to turn into a steam and gas state of any conductive material. Undoubtedly, the formation of particles of the evaporated material and their energy saturation proceed from the steam or gas state [8].
Obtaining self-organized nanostructures using pulsed plasma has its advantages: the ability to regulate the energy of a single pulse allows to increase the proportion of particles with nanoparticles, the use as a reaction medium – dielectrics helps to stabilize the emerging nanoparticles components of the medium. In turn, the surface tension energy at the interface between two immiscible liquids is an additional energy to the pulse plasma energy.
For nanostructuring on the interfacial areas of the electrodes were taken cadmium metal 98,90 % purity. Dispersion of cadmium proceeded faster in comparison with other metals. Cadmium nanostructuring was carried out in water – toluene microemulsion, using a high-speed magnetic stirrer, as a result of which two fractions were obtained-heavy (sediment) and light (which surfaced on the emulsion surface). In the obtained samples, the light fraction was carefully separated from the emulsion surface, and the sediment was filtered and dried well.
The dispersed powders obtained in the microemulsion were analyzed on the Rigaku Geigerflex X-Ray Diffraktometer with CuKα – radiation. Sediment diffractogram, cadmium dispersion product contained reflexes (002), (100), (101), (102), (103) and (112) (Fig. 1) relating to metal cadmium. The crystal lattice of cadmium is hexagonal, with options alit = 2,9793 A, сlit = 5,6181 A literature data. The experimental lattice parameters of the particles of the cadmium precipitated emulsion (water-toluene): aexp = 3,176757 A, сexp = 5,46586 A). Was also discovered the diffraction lines of the cadmium oxide CdO reflexes (220) (311) cubic lattice parameter, aexp = 4,73477 A, (a lit = 5,273 A). Defined diffraction lines CdCO3 with reflexes (012), (104), (202), (024), (115), (102) having a trigonal crystal lattice with the parameters of aexp = 4,76469 A and cexp = 13,84346 A, literature data (alit = 4,930 A, с lit = 16,27 A) and the crystal lattice of Cd(OH)2 hexagonal reflexes (001), (100), (102) and (110), where aexp = 3,51904 A, сexp = 5,18070 A (alit = 3,496 A, сlit = 4,702 A). The half-width of the reflexes identified by using the Scherrer formula, for each phase, the calculated particle sizes: D(Cd) = 15.7 nm; D(CdO) = 17 nm; D(CdCO3) = 17,64 nm; D(Cd(OH)2) = 16 nm.
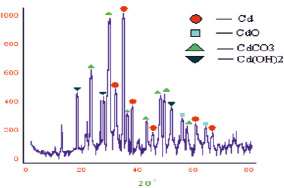
Fig. 1. Diffractogram of cadmium nanoparticles precipitated, in the system (toluene-water)
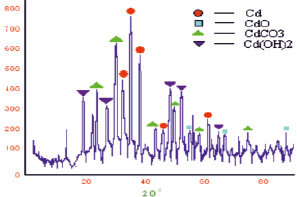
Fig. 2. Diffractogram of cadmium nanoparticles that floated to the surface emulsion (toluene-water)
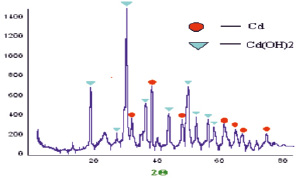
Fig. 3. Diffractogram of cadmium nanoparticles obtained in water
For a light fraction of the cadmium sample, the same reflexes as for the heavy fraction were found, but here the diffraction lines are less pronounced. The parameters of the crystal lattice of light fraction for cadmium metal is equal to and аexp = 2,89963 A, with сexp = 5,65593 A. The crystalline lattice of CdO cubic parameter, aexp = 4,5786 A. Cadmium carbonate CdCO3 has a trigonal crystal lattice settings, aexp = 4,08286 A, сexp = 16,7953 A. The parameters of the crystalline lattice Cd(OH)2, aexp = 3,74282 A, сexp = 4,71737 A (alit = 3,496 A, сlit = 4,702 A) hexagonal structure. The particle size was: D(Cd) = 19,64 nm; D(CdO) = 22,3 nm; D(CdCO3) = 21,5 nm; D(Cd(OH)2) = 16,3 nm.
Processing of diffraction patterns of the synthesized nanoparticles of cadmium in water show that cadmium particles have a hexagonal crystal lattice with the lattice parameters: aexp = 3,01199 Å, cexp = 5,73735 Å (Fig. 3). Crystalline lattice nanostructures Cd(OH)2 is monoclinic with the parameters of aexp = 5,93525 Å, bexp = 10,38348 Å, cexp = 3,51046 Å, and literature data γCd(OH)2 alit = of 5.63 Å, blit = 10,18 Å, clit = 3,40 A. The particle size of which amounted to: D(Cd) = 15,14 nm; D(Cd(OH)2) = 18,25 nm. Processing of diffraction patterns of nanoparticles of cadmium synthesized in pure toluene show that the particles of cadmium are hexagonal crystal lattice settings, aexp = 2,9927 Å, cexp = 1,6352 Å, where literature data alit = 2,9793 Å, clit = 5,6181 Å. The crystal lattice cubic CdO, option, aexp = 4,7273 Å (Fig. 4). The size of the particles is: D(Cd) = 20,8 nm; D(CdO) = 12 nm.
The results of processing the diffractogram show changes in the parameters of crystal lattices in comparison with the literature data, which are due to the conditions of pulsed plasma [8] and the processes occurring on the interphase surface in microemulsion (water-toluene).
Cadmium dispersion products in toluene and in microemulsion (water-toluene), using energy and coolant, in the form of dispersed powders are subjected to electron microscopic analysis using a translucent electron microscope TEM JEOL-2000FX. In fig. 5. TEM images of cadmium nanostructures obtained a) in pure toluene, b) in microemulsion (water-toluene) using the energy of PPL are presented. The results of the analysis of TEM image, in the case of nanostructuring cadmium in pure toluene, showed the formation of two phase-spherical nanoparticles of metal cadmium and cadmium oxide (Fig. 5. a) When cadmium nanostructuring in microemulsion (water-benzene), cadmium nanowires, cadmium oxide and cadmium hydroxide are formed (Fig. 5. b).
Thus, when nanostructuring metal cadmium, changing the composition of the medium is very important. Metal cadmium, cadmium oxide and cadmium hydroxide nanowires are formed only at the boundary of two immiscible toluene and water liquids. Nanoparticles of cadmium metal, cadmium oxide and cadmium hydroxide are formed in pure water and toluene.
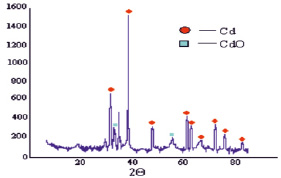
Fig. 4. Diffractogram of a sample of cadmium nanoparticles obtained in pure toluene
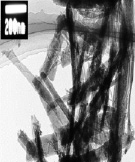
a) b)
Fig. 5. TEM images of cadmium nanostructures obtained a) in pure toluene b) in a microemulsion (water-toluene), using of energy PPL
Conclusion
Was first carried out work on the dispersion of metallic cadmium, with the use of energy PPL in the environment of toluene in water and in microemulsion (water-toluene), where we have used the total energy PPL and the energy of surface tension on the interfacial surface. The phase composition of the obtained compounds is studied and determined their size.
The results of processing diffractograms show changes in the parameters of crystal lattices of synthesized compounds. For products obtained in microemulsion, the particle size of metallic cadmium, cadmium oxide, cadmium carbonate and cadmium hydroxide is much smaller than the particle size obtained in pure toluene, using the energy of PPL. Thus, the dispersion of cadmium in the microemulsion under the influence of the total energy of pulsed plasma and the energy of surface tension is a significant reduction in the particle size.
Formation of cadmium nanowires, cadmium oxide and cadmium hydroxide occur due to the presence of an interphase surface, which plays the role of a flat substrate on which two-dimensional layers of cadmium are formed, as well as its oxide and hydroxide with further folding them into nanowires. In the known methods of synthesis of cadmium-based nanowires, it is required to use auxiliary precursors in the form of pores, layered compounds, etc., which contaminate the target products. In our case, ~90 % of the energy supplied to the electrodes is spent on nanostructuring the material of the electrodes with encapsulation of its resulting nanostructures.

