Iodine is one of the most indispensable nutrients and is a unique (by biological activity) medicine in a pharmaceutical aspect. It is mainly used as part of an inclusion complex with polyvinyl alcohol or polyvinylpyrrolidone rather than in an individual form [1]. Iodine inclusion in such a synthetic polymer matrix allows reducing its concentration in the corresponding pharmaceutical and its toxic effects on the body, achieving a pronounced prolonged effect, and increasing the efficiency. Meanwhile, iodine immobilization in a biologically active polymer matrix, e.g. in chitosan, along with the foregoing, will make it possible to obtain a biodegradable dosage form with better biocompatibility with human dermal tissues as compared to iodine adducts with synthetic polymers [2, 3]. This determines the relevance of studying of the interaction of chitosan with iodine to find conditions for stabilizing this compound in the aminopolysaccharide matrix.
The following ways to prepare iodine-containing chitosan derivatives with a fixed iodine amount in the polymer matrix are known: addition of molecular iodine and iodic acid salts to a chitosan solution [3], mixing of polymer and iodine solutions [4-6], and restricted swelling of a solid form of chitosan (film, flakes) in iodine-containing solution [7, 8]. However, the iodine-chitosan complexes thus formed are thermally and kinetically unstable (their decomposition at 20-25 °C proceeds in ~5-10 days). R.Kh. Murzagildina [9] describe modification of chitosan films in the vapor above crystalline iodine to form film samples of iodine-containing chitosan with increased antibacterial activity. In order to obtain iodine-containing derivatives, O.A. Belyakova [10] modified chitosan powder (the source material to produce film samples) in the vapor above an iodine-containing medium. Aqueous and water-alcohol solutions of iodide and iodine-iodide, crystalline iodine were used as such media. In Ref. [11] it has been shown that the chitosan-iodine complexes obtained in this way are stable to heat treatment and storage (longer than a year).
Chitosan belongs to the class of optically active (chiral) polymers. The presence of asymmetrically substituted carbon atoms (chiral centers) in the chitosan macromolecules leads to the appearance of the optical activity of one unit and, as a consequence, that of the macromolecule as a whole [12, 13]. Chitosan modification in iodine-containing vapor may affect the general chiral structure of the polymer sample and, accordingly, may reflect on both the chiro-optical properties and the biological activity of the preparations obtained on its basis. For example, the difference in the optical activities of films made of chitosan of different molecular weight and modification is known to strongly affect their bacteriostatic action, until full loss of bactericidity [13, 14].
The aim of the present work was the preparation, spectroscopic analysis and evaluation of the biological activity of powdered chitosan-iodine complex obtained by polymer modification in iodine-containing vapor.
Materials and methods of research
The chitosan-iodine complex was obtained by modifying chitosan powder in the vapor phase of iodine-containing media by the method described elsewhere [15]. A sample of chitosan hydrochloride with a viscosity average molecular weight of 38 kDa, a deacetylation degree of 80 mol %, and a bulk density of 0.78 g/cm3 (Bioprogress Ltd., RF) was used. The vapor formed above crystalline iodine, 0.63 and 1.26 % aqueous iodine-iodide solutions, and 5 and 10 % water-alcohol iodine-iodide solutions was used as the sorbate. Aqueous and water-alcohol solutions of potassium iodide (KI) were used for comparison.
Experimental part
Obtaining of the chitosan-iodine complex
To obtain the chitosan-iodine complex, an air-dry polymer powdered sample was kept in the vapor formed above the surface of an iodine-containing media at 20 ± 2ºC to achieve an equilibrium sorption degree (CS(t∞), wt %). The sorbed vapor amount was measured gravimetrically, by weighing the polymer sample on an analytical balance OHAUS Discovery DV215CD (accuracy ±0.0001 g). The modified samples were stored under standard conditions: room temperature and normal atmospheric pressure.
Spectroscopic analysis
Absorption spectra were recorded on a UV2550 Shimadzu spectrophotometer (Japan) in a wavelength range of λ = 250–750 nm with an accuracy of 0.3 nm in quartz cuvettes 1 cm wide relative to water. For analysis, aqueous solutions of polymer samples were used with concentrations of 0.01 and 0.1 g/dL. The IR spectra of powdered sanples were recorded on an FSM 1201 (RF) Fourier spectrometer with an accuracy of 0.1 cm-1.
Optical rotation dispersion
Optical rotation dispersion (ORD) spectra were recorded on a PolarAr3001 spectrophotometer, Optical Activity Ltd (GB) in a range of λ = 400–600 nm at 25 °C. A temperature-controlled quartz glass cuvette 1 dm long was used, the solution concentration was 0.1 g/dl. The experimental conditions were standard, the error in measuring rotation angles did not exceed ±0.002 deg. For the mathematical processing of ORD experimental curves, the Drude equation was applied:
[α] = K/(λ2 – λ02),
where [α] is the specific optical rotation, K and l0 are the rotational and dispersion constants, respectively.
Assessment of biological properties
The bactericidal activity was assessed by the in vitro diffusion method in agar relative to strains of the test cultures of Staphylococcus aureus (209 P) and Escherichia coli (113-13). To examine the kinetics of iodine release from the chitosan-iodine complex, a semipermeable cellophane membrane and model media simulating biological fluids were used: 0.1 M HCl solution with pH 1.1 (gastric juice), acetate buffer with pH 5.8 (saliva), borate buffer with pH 7.6 without and with the addition of pancreatin as an enzyme (the small intestine medium). The experiment was carried out at 37 °C during 4 h. The iodine released into the aqueous phase was analyzed spectrophotometrically, by its maximum absorption at λ = 600 nm, using water-soluble starch as an indicator, the released iodine concentration was estimated from a calibration line. Cytotoxicity was evaluated by the in vitro method of culturing a test cell culture in a nutrient medium supplemented with aqueous solutions of some preparations based on iodine, chitosan and the chitosan-iodine complex. The MA-104 macaque embryo kidney epithelial cell line was used from the collection of the Virology Research Institute of the Russian Academy of Medical Sciences (RF). Cell adhesion and proliferation were observed on an inverted BiolamP microscope (RF).
Research results and discussion
It was established earlier that air-dried chitosan powder had a high affinity for iodine-containing vapor [10]. For example, when sorption of the vapor formed above an aqueous and water-alcohol solutions of potassium iodide with no iodine added, the maximum achievable values of the sorption degree are ~50–90 wt %. Iodine addition to the vapor-generating medium leads to an increased sorption degree (by 3–5), up to CS(t∞) ≈ 250–300 wt %. An intermediate value of CS(t∞) ≈ 100 wt % is observed during sorption of the vapor above crystalline iodine. In this case, the sorption kinetics of the vapor of iodine-containing media by chitosan powder is described by anomalous sorption curves, indicative of structural rearrangements of the polymer matrix [16, 17] and the formation of a new chemical substance [10].
As the vapor of iodine-containing media is sorbed, the color of the chitosan powder changes from light beige to dark brown and even black. The aqueous solutions of the source chitosan sample are colorless, while those of the modified one show red-violet staining. Besides, the solubility of the sample modified in the vapor of iodine-containing media changes. The source chitosan hydrochloride is readily soluble in water up to the formation of highly concentrated solutions (≥ 4–5 g/dl). The water solubility of the modified chitosan decreases, but solubility in a 60–70 % aqueous ethanol solution is acquired.
To establish the chemical composition of the chitosan adducts with iodine-containing vapor, freshly obtained samples and those stored for a year were analyzed by electron and IR spectroscopy.
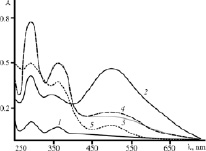
Fig. 1. Electronic spectra of aqueous I2 + KI solutions (1) and solutions of the chitosan hydrochloride sorbed the vapor over iodine-iodide aqueous solution (2, 5), water-alcohol iodine-iodide solution (3) and crystalline iodine (4): the source sample (2-4) and that stored for a year (5)
The spectrum of the aqueous I2 + KI solution (comparison solution) exhibits two maxima at wavelengths of ~290 and ~350 nm, corresponding to the absorption bands of I3¯ and IO3¯ ions, respectively (Fig. 1, curve 1). The spectrum of the aqueous solution (of a comparable concentration) of the chitosan hydrochloride powder sorbed the vapor over the aqueous iodine solution is also characterized by the absorption maxima of I3¯ and IO3¯ ions (curve 2). However, for the spectrum of the iodinated chitosan solution, a bathochromic shift of the maxima of the spectral bands by ~3–5 nm with simultaneous hyperchromic effect is characteristic. In addition, a third absorption band with a maximum at ~500 nm is present in the spectrum of the solution of the polymer modified in iodine-containing vapor. It is believed that the absorption maximum at ~500 nm for the I2-chitosan complex is due to the formation of exciton bonding between polyiodide ions and the macromolecule with charge transfer [4].
Similar spectra (with three intense absorption bands at λ = ~290, ~350 and ~500 nm) were also observed for the solution of the chitosan hydrochloride powder absorbed the vapor over water-alcohol iodine-iodide solution and crystalline iodine (Fig. 1, curves 3 and 4). In this case, the hyperchromic effect of the maxima of the absorption bands of I3¯ and IO3¯ ions is significantly higher compared with both the solution of the sample kept in the vapor above the aqueous iodine-iodide solution (curve 2) and the iodine-iodide solution with no chitosan (curve 1). It should be noted that the spectrum of the chitosan adduct with iodine-containing vapor stored for a year has the same absorption maxima as the solutions of the freshly modified sample (curves 2 and 5). The intensity of the peaks at λ ~290 and ~350 nm increases, but that at λ ~500 nm decreases. Nevertheless, the qualitative composition of the I2-chitosan complex does not vary, which confirms its stability when stored under standard conditions.
It was found that when the modified sample was heated at a temperature below the thermodestruction point of the polymer, the chitosan-iodine complex was stabilized. E.g., for an aqueous solution of the chitosan hydrochloride sorbed the vapor over aqueous iodine-iodide solution and heated at 130 °C for 15–60 min, a significant increase in the intensity of the absorption bands at λ ~290 and 350 nm is characteristic, as well as a small increase of that at ~500 nm in comparison with the reference solution of the non-heated modified sample. When further heat treatment (120–150 min), the intensity of these absorption bands decreases, but the optical density values of the reference solution was not achieved. According to the literature data, during the heat treatment of chitosan, acylation of its amino groups and cross-linking of the chains may occur [18], the “hairpin” structure of the macromolecule may be stabilized [19]. Since the intramolecular hydrogen bonds formed under these conditions are sufficiently stable, they could be responsible for the stabilization of the chitosan-iodine complex during its heat treatment.
IR spectrometry has detected changes in the deformation and stretching vibrations of functional groups and other macrochain fragments caused by diffusion of the iodide-containing vapor into the chitosan matrix. For example, in the spectrum of the source chitosan hydrochloride powder, intense absorption bands with maxima at ~1,600 and ~1,380 cm-1 are present, corresponding to antisymmetric deformation vibrations of the protonated amino group –NH3+ and deformation vibrations of the hydroxyl group –OH (Fig. 2, curve 1). In addition, an intense absorption band at ~1,060 cm-1, belonging to the stretching vibrations of the C–O, C–N, and C–C bonds of the glucopyranose ring, was recorded in the IR spectrum of the source sample [20]. After sorption of iodine-containing vapor by the polymer, the maximum of the absorption band at ~1,600 cm-1 in the IR spectrum shifts toward shorter wavelengths (by ~100 cm-1), and the peak at ~1,380 cm-1 disappears (curve 2). The band intensity at ~1,050 cm-1 significantly reduces, which may be due to destruction of the network of intramolecular and intermolecular hydrogen bonds. All this testifies to the interaction of the sorbate vapor with the functional groups of chitosan during diffusion of the iodine-containing component and to some physicochemical coordination of iodine in the polymer matrix [21].
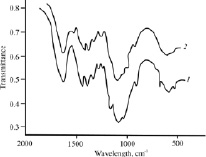
Fig. 2. IR spectra of the source powder of chitosan hydrochloride (1) and that modified in iodine-containing vapor (2)
Similar spectral (UV, IR) characteristics were also obtained in other works [5, 6] when studying of the complexation of water-soluble and acid-soluble chitosan with iodine in water and in an aqueous solution of nitric acid. The authors explain the results obtained by the formation of a host-guest chitosan-iodine complex consisting of an internal polyiodide chain surrounded by an ensemble of interconnected hydrogen bonds among the chitosan chains. Both hydroxyl and amino groups of the aminopolysaccharide were suggested to be the main centers to hold the polyiodide chain inside the chitosan matrix [5]. The results of our work confirm this assumption for the system “chitosan-iodine-containing sorbate vapor” as well.
For a more detailed study of the spatial structure of the powdered chitosan-iodine complex, its specific optical rotation [α] was measured, since optical activity is extremely sensitive to any, even subtle, structural changes in a polymer system. The sign and integral intensity of ORD depend on the structure and conformational features of the macrochains. For comparison, a source sample of chitosan hydrochloride and the chitosan adduct with iodine obtained by adding I2 to the polymer solution were taken.
The ORD curve of the aqueous solution of the source powder of chitosan hydrochloride lies in the region of negative values of specific optical rotation, has neither a maximum, nor a minimum, nor an inflection point, i.e. belongs to the normal type (Fig. 3, curve 1). Dispersion curves of this kind are typical for the polysalt form of chitosan, either in solution or in a condensed film form. In particular, an analogous type of monotonous ORD curves with levorotation was observed for chitosan solutions in acetate buffer [12, 22], acetic acid [23, 24], as well as in chitosan acetate films [12, 13]. Analogous [α] = f (λ) dependences also take place for chitosan films in the polybasic form obtained from acetic acid solutions of the polymer with subsequent transferring to the polybasic form using NaOH [13]. Iodine addition to the aqueous solution of chitosan hydrochloride leads to the appearance of an anomalous ORD curve characterized by both positive and negative values of specific optical rotation with [?] sign inversion at λ ~480 nm (curve 2). In this case, in the ranges λ < ~450 nm and λ > ~530 nm, the absolute value of optical rotation is higher than that of the solutions of the source chitosan hydrochloride. The sample sorbed iodine vapor (equivalent to the introduced I2 additive) is also characterized by anomalous dispersion. However, the [α] = f (λ) dependence twice changes the [α] sign at λ ~465 and ~585 nm, passes through maxima and is characterized by even higher absolute values of specific optical rotation (curve 3).
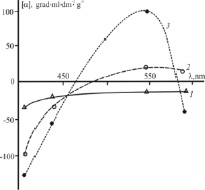
Fig. 3. ORD of the aqueous solutions of the source chitosan hydrochloride (1), the same solution with iodine addition (2), and the sample sorbed the vapor over crystalline iodine (3). The iodine addition to the chitosan solution is equivalent to the value of the sorption degree of the iodine-containing vapor over crystalline I2
For the theoretical isolation of spectral bands of optically active chromophores and for obtaining additional parameters of electronic transitions in the macromolecules, the single-term Drude equation was used. Mathematical processing of our experimental ORD curves has revealed that the optical rotation of the source and modified (I2 addition, iodine-containing vapor sorption) chitosan hydrochloride in aqueous solution is due to the contribution of optically active chromophores with different positions of their absorption bands (Table, the dispersion constant ?0). The contribution of the space-electronic transition to the total optical activity of the source and modified polymer system also differs (the rotational constant K).
The source chitosan hydrochloride in aqueous solution is characterized by one space-electron transition in the UV region (Table). I2 addition to the polymer solution shifts the absorption wavelength of the optically active chromophores into the visible region. The rotational constant changes sign and increases by absolute value, although not very significantly. For the chitosan-iodine complex obtained by modifying the polymer in the vapor over crystalline iodine, even higher values of ?0 and K are observed (the treatment was carried out using the left branch of the dispersion curve). The results of our mathematical processing of the ORD spectra are in satisfactory agreement with the experimentally obtained electron spectra of the chitosan adducts with iodine-containing vapor, in particular, with the electronic transition with the absorption maximum at ? ~ 500 nm (Fig. 1).
It should be noted that the chitosan-iodine complex obtained by mixing the solutions of the polymer and I2 + KI in acetate buffer showed an electron spectrum with an exciton absorption maximum at 500 nm and mutually separated bands with opposite signs (+, –) at 460 and 540 nm in the circular dichroism spectrum [4]. Therefore, the recorded [?] = f (?) spectrum of the aqueous solution of the chitosan-iodine complex, the calculated therefrom value of ?0 ~ 530 nm and published data [4] confirm the possibility of exciton dissociation with charge transfer and the exciton-bound interaction of chitosan and polyiodide chains.
Our evaluation of biological activity showed that the powdered chitosan-iodine complex in therapeutic doses had no bactericidal action against both gram-positive (S. aureus) and gram-negative (E. coli) microorganisms. Moderate bactericidal activity was observed only when the therapeutic concentration of the drug was doubled.
Values of the rotational and dispersion constants in the Drude equation for the source and modified chitosan hydrochloride in aqueous solution
|
Aqueous solution of chitosan hydrochloride |
Drude equation constants |
|
|
Dispersion constant λ0 (nm) |
Rotational constant К·10-6 |
|
|
Source polymer |
300 ± 5 |
–2.07 ± 0.05 |
|
Source polymer + I2 additive |
450 ± 5 |
2.12 ± 0.14 |
|
Chitosan-iodine complex (the polymer sorbed iodine-containing vapor) |
530 ± 15 |
6.73 ± 0.13 |
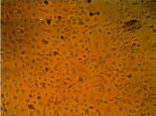
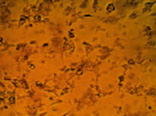
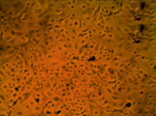
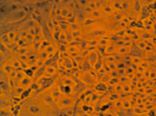
Fig. 4. MA-104 cell culture after 2 days of cultivation in the standard nutrient medium (a) and that supplemented with aqueous solutions of our preparations based on iodine (b), chitosan hydrochloride (c) and the chitosan-iodine complex obtained by polymer sorption of the vapor over crystalline iodine (d). The concentration of the medicines administered corresponded to the therapeutic dose
When studying of iodine release from the modified chitosan matrix, it was established that the release of the medicinal substance in a 0.1 M HCl solution was prolonged, iodine release began immediately after the start of the experiment. In acetate buffer, the release of iodine is limited and begins only after 3 h of sample exposure in this model medium. In the borate buffers without or with the addition of pancreatin, no iodine release from the chitosan-iodine complex was observed.
Cultivation of the epitheliocyte cell culture of the macaque embryo showed no cytotoxicity for the chitosan modified in iodine-containing vapor. E.g., the adhesion and proliferation of MA-104 culture cells in the nutrient medium supplemented with solutions of chitosan hydrochloride and the chitosan-iodine complex occurred at the same time as in the reference medium. On the 2nd day of observation the cell culture in the standard nutrient medium formed a complete monolayer (Fig. 4 a). In the nutrient medium with the preparations of chitosan hydrochloride (Fig. 4 c) and the chitosan-iodine complex (Fig. 4 d), the monolayer was formed more slowly and small fields with the absence of cell growth in separate areas were observed. Nevertheless, on the 3rd day of cultivation, the formation of a full epitheliocyte monolayer was observed. In the nutrient medium supplemented with I2 solution, mass death of MA-104 cells was observed (Fig. 4 b). Only single cells showed adhesion, but did not proceed to division.
On the basis of our studies it can be concluded that the preparations based on the chitosan-iodine complex, as well as on the source polymer, exhibit no cytotoxicity. This fact testifies to the iodine in the chitosan matrix being in a bound state, weakly diffusing into the culture medium, and not adversely affecting the adhesion and proliferation of epithelial cells.
Conclusions
Our experiments and published data allow us to assume the following mechanism for the chitosan-iodine complex formation while the polymer is modified in the vapor of an iodine-containing medium. At the first stage, the molecules of the sorbed components of the vapor phase condense on the surface of the polymer powder particles, and then diffuse into their bulk. As the iodine-containing vapor sorption does not obey Fick’s law [10], the different rates of conformational changes in the macromolecules when the amorphous phase of the polymer is filled with sorbate molecules and competing diffusion lead to a break of the intermolecular and intramolecular hydrogen bonding in the polymer and increase the segmental mobility of macromolecular chains [16, 25, 26]. Iodine interacts with the functional groups and other fragments of the microchains and a new chemical substance is formed (Fig. 1-3). Taking into account the fact that the chitosan macromolecules take on a helical conformation in highly ordered regions [19], it was assumed that iodine is embedded into the internal cavity of the helical regions of the mobile chitosan macromolecule to form a charge transfer complex. The hydroxyl and amino groups of chitosan serve the centers of iodine retention inside the macromolecular chain. The structural difference between our chitosan-iodine complex and the classical polysaccharide-containing amyloiodine clathrate is that the inner layer of the two-layered cylinder is represented by shorter polyiodide chains and the outer layer is by a “loose” polymeric helix.
Evaluation of the biological activity and cytotoxicity of our chitosan-iodine complex indicates the promise of its use in the design of iodine-containing preparations with controlled release, which would not adversely affect the microflora in the living organism.
The results of the work were obtained with the financial support of the Russian Science Foundation grant No 17-73-10076 “Chiral polymeric matrices: preparation, physicochemical properties, interaction with bioobjects”.

