Survival of staphylococci on the nasal mucosa critically depends on their antagonistic activity, while the presence of factors of intermicrobial interaction is one of the important selective advantages of staphylococcus persistent. Factors of intermicrobial interaction, of which the most significant are enzymes (peptidoglycan lyzing enzymes) and non-enzymatic factors of peptide origin – bacteriocins [7], give a selective advantage to staphylococci during colonization of the open ecological niches of the host organism [4].
Interestingly, staphylococci isolated from the nasal mucosa from the blood in chronic infections are often characterized by low virulence, reduced hemolytic activity, decreased growth rate, the shape and size of the colonies, autotrophic feeding, altered metabolism, and impaired peptidoglycan formation [1, 3]. As shown by us earlier, bacteria isolated from foci of purulent-inflammatory diseases of soft tissues are more hydrophilic than bacteria that live (parasitizing) on mucosal surfaces [9].
Moreover, the chemical structure of many intermicrobial interaction factors remains unknown, and therefore empirically they can be combined into a peptidoglycan lyzing factor (PLF), combining chemical compounds of various types (peptidoglycan enzymes and non-enzymatic factors (bacteriocins)).
Therefore, the aim of the study was to determine the chronological conjugation of the growth kinetics of staphylococci with the dynamics of peptidoglycan lyzing factor release and physicochemical properties.
Materials and methods of research
The material of the study was 66 strains of clinical isolates of staphylococci (S. aureus-20, S. epidermidis-10, S. haemolyticus-24, S. hominis-12) isolated from the nasal mucosa and peptidoglycan-lysing activity. In the work, cultures adapted to the medium and synchronized cultures of staphylococci were used. Every hour, the (OD) cultures were recorded on a spectrophotometer (l = 540 nm) and the specific growth rate (m, h-1) [6] was determined.
Isolation of peptidoglycan lyzing factor. In parallel with the measurement of the reproductive potential of staphylococci, the diameter of diffusion zone of peptidoglycan lyzing factor was measured. As a marker for the presence of peptidoglycan in the medium, the strain Micrococcus lysodeikticus N2665, GISK im. Tarasevicha (the presence of a more “loose” peptidoglycan mesh in Micrococcus lysodeikticus, promotes high lysis of cell wall lysozyme).
On the surface agar (NPO “Nutrient mediums”, Makhachkala, Russian Federation) with a dots with d = 5 mm, daily culture of staphylococcus was applied, cultivated in +37 °C. Cultures were lysed (chloroform pairs, 5 min) and filled with a continuous lawn of M. lysodeikticus. On the following day, the diameter of the growth retardation zones was measured (they corresponded to the diameter of the diffusion zones of peptidoglycan lyzing factor).
Sowing on agar plates, a producer of peptidoglycan lyzing factor (S.epidermidis SE711), was performed by inoculation with streaks at intervals of 20 mm, after 24 hours of growth, the agar stripe was cut from the spirit of the sides from the grown colonies 5 mm from the edge of the colony and width of 10 mm. Agar plates 1×50 mm were filled with 50 ml of 0.1 % AcOH solution and dispersed on a magnetic stirrer. The resulting extract was further clarified (Durapor filters, 0.22 nm) and separated by reversed-phase HPLC.
Analytical study of agar extracts (containing peptidoglycan lyzing factor) was carried out on a liquid chromatograph “Smartline” (Knauer, Germany). We used Waters chromatographic columns packed with μ-Bondapak sorbent (C18, 5 μm, 3.9×300 mm). For reversed-phase high-performance liquid chromatography (HPLC), the preparations were diluted (1: 100) with 0.01 % AcOH solution, layered 5 μl per C18 column (3.9×300 mm). The elution was carried out with a linear gradient (acetonitrile + 0.1 % TFA / H2O + 0.1 % TFA) for 10 min, the detector response was fixed at two wavelengths of 220 nm and 280 nm.
Measurement hydrophobicity of bacteria. To measure the degree of hydrophobicity of the surface of bacterial cells, a two-phase separation of bacterial suspension in the system “polyethylene glycol PEG 6000-dextran T500” was applied [2]. The optical densities of the upper (PEG 6000, Upsala (Sweden)) and lower (dextran T500, Upsala (Sweden)) phases were measured on a spectrophotometer at a wave length (λ = 540 nm). The hydrophobicity was expressed as a hydrophilic-lipophilic balance (HLB = Lg [ODPEG / ODDextran]) of the surface [8]. For standardization of results, they were expressed in absorbance units (AU).
Statistical analysis. The obtained results were subjected to statistical processing by the methods of variation statistics [5].
Results of research and their discussion
At the first stage of the study, clinical strains of staphylococci were selected, with growth on a solid nutrient medium peptidoglycan releasing factor (peptidoglycan lyzing factor). As a result, 20 cultures of S. aureus and 46 cultures of coagulase-negative staphylococci (10-S.epidermidis, 24-S. haemolyticus and 12-S. hominis) were selected.
In the cultivation of staphylococci on a solid nutrient medium, the time for the initiation of peptidoglycan lyzing factor isolation over the M. lysodeikticus growth inhibition zone was noted. For S. aureus, the first signs of the release of the factor (peptidoglycan lyses activity) were observed after 6 hours of growth, with the formation of a characteristic lyses zone of M. lysodeikticus (4.5 ± 0.3 mm). Later, the growth dynamics of the diameter of the M. lysodeikticus lysis zone were noted. By 8 hours of growth, the diameter of the M. lysodeikticus growth inhibition zone increased by 29 % (5.8 ± 0.3 mm), and after 24 hours of growth, it was 84 % (8.3 ± 1.0 mm). Coagulase-negative staphylococci were characterized by more pronounced peptidoglycan expression of lysis activity. Thus, the diameter of the diffusion zone of PLP for S. epidermidis already by 6 hours of growth was (3.1 ± 0.3 mm), by 8 hours it increased by 19 % (3.7 ± 0.4 mm), and after 24 hours by 106 % (6.4 ± 0.7 mm). But the largest increase in the diameter of the M. lysodeikticus lyses zone was noted in the cultivation of S. haemolyticus clinical cultures. If by 6 o’clock growth the diameter of the diffusion zone of the FLP was 3.3-0.5 mm, by 8 hours it increased by 45 % (4.8 ± 0.9), and after a day of growth by 142 % (8.0 ± 1.0). The mean values of 18-hour staphylococcal cultures for hydrophobicity for Staphylococcus-releasing PLF: S. hominis (-1.725 ± 0.17 AU), S. haemolyticus (-1.317 ± 0.12 AU), S. epidermidis (-1.024 ± 0.13 AU), S. aureus (-0.893 ± 0.15 AU).
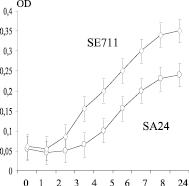
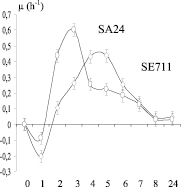
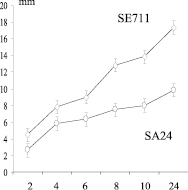
At the next stage of the study, two clinical isolates of staphylococci differing by the maximum specific growth rate of S. aureus SA24 and S. epidermidis SE711 with high expression peptidoglycan lyzing factor (Figure), extraction of peptidoglycan lyzing factor was carried out.
In two cultures with the highest dynamics of peptidoglycan isolation, fractionation by reversed-phase chromatography (HPLC) For preparation of reversed-phase liquid chromatography, preparations of agar extracts containing peptidoglycan lyzing factor were diluted (1: 100) with 0.1 % acetic acid solution, layered (3.9×300 mm), elution was carried out with a linear gradient (acetonitrile + 0.1 % TFA / H2O + 0.1 % TFA) for 10 minutes, the detector response was fixed at two wavelengths of 220 and 280 nm (Table).
In the gradient elution mode, the maximum efficiency of the agar extract separation was achieved at a ratio of 60:40 (acetonitrile + 0.1 % TFA / H2O + 0.1 % TFA), with an elution rate of 1 ml / min. Fractionation was carried out in duplicate. At the first separation, two fractions were obtained with a retention time of 2.9 and 4.7 minutes. Lyophilisates of all peptidoglycan fractions did not have any activity (lysed M. lysodeikticus cells in a test tube, in control lysozyme solution). With the second separation by reverse phase HPLC on a C18 column, 2 fractions were obtained with a retention time of 2.6 and 3.7 minutes.
Interestingly, cultures in which the peptidoglycan lyzing factor was released up to 6-8 hours of cultivation, according to the inhibition of M. lysodeikticus growth, but after a day of growth we did not observe this activity. Perhaps this is due to the inactivation of the released peptidoglycan lyzing factor.
a) b)
c)
Chronological conjugation of morpho-physiological properties of staphylococcus producers of peptidoglycan lyzing factor (PLP) with a diameter of the lysis zones. On the abscissa axis: cultivation time (hour); on the ordinate axis: a) – biomass OD; b) is the specific growth rate μ (h-1); c) – diameter of the lysis zones of Micrococcus lysodeikticus (mm); SE711 – Staphylococcus epidermidis and SA24 – Staphylococcus aureus producers of peptidoglycan lyzing factor (PLP)
Fractionation of agar extract
|
Fractions |
Fractionation 1 |
Fractionation 2 |
||||||
|
Wavelength, λ |
220 nm |
280 nm |
220 nm |
280 nm |
||||
|
Peaks |
1 |
2 |
1 |
2 |
1 |
2 |
1 |
2 |
|
Max. RT |
2.87 |
4.68 |
2.87 |
4.70 |
2.63 |
3.67 |
2.63 |
3.68 |
|
Start RT |
2.77 |
4.10 |
2.77 |
4.03 |
2.48 |
3.40 |
2.45 |
3.42 |
|
End RT |
3.00 |
5.28 |
2.98 |
5.28 |
2.78 |
4.08 |
2.78 |
4.08 |
|
Area |
2.63 |
11.04 |
2.23 |
11.68 |
1.91 |
3.59 |
1.69 |
3.46 |
|
Heigh |
29.39 |
34.68 |
25.42 |
34.49 |
23.55 |
14.24 |
19.91 |
14.01 |
|
Width |
0.08 |
0.24 |
0.08 |
0.25 |
0.07 |
0.23 |
0.24 |
0.23 |
Note: Max. RT (min) is the peak hold time; Start RT (min) – start time of peak integration; End RT (min) – the retention time of the end of peak integration; Area (mAU * min) – area of the peak; Heigh (mAU) – peak height; Width (min) – peak width.
The presented experimental data indicate that one of the characteristics of the more characteristic coagulase-negative staphylococci, in contrast to S. aureus, is the hyper production of the peptidoglycan lyzing factor. For staphylococci with a low specific growth rate, hyper production of the peptidoglycan lyzing factor and the higher hydrophobicity of the cells are characteristic. Perhaps this is one of the factors of inter-microbial antagonism contributing to the maintenance in the population of clones with a low growth rate and one of the criteria for selecting a morphotype with special physicochemical properties of the surface. The explanation of this phenomenon is probably associated with a different survival strategy for CNS and S. aureus on mucous membranes. An unsuccessful attempt to isolate the peptidoglycan by the HPLC method is due to the inactivation of the phosphate factor present in the agar and, to a greater extent, in the broth, the main component of which is the sprat hydrolyzate. This, to some extent, can explain the manifestation of low activity in the period of 6-8 hours of growth of staphylococci and the lack of activity after 24 hours of growth.
Conclusions
A comparative analysis of the dynamics of the increase in the zone of inhibition of the growth of micrococcus showed that the largest diameter was observed in S. aureus, and among the coagulase-negative staphylococci, S. haemolyticus showed the greatest dynamics of growth of the zone of inhibition of M. lysodeikticus cells growth. By the peptidoglycan lyzing factor expression criterion, all clinical isolates could be arranged in a gradient series: S. aureus > S. haemolyticus > S. epidermidis > S. xylosis > S. hominis. By high hydrophobicity in a gradient series: S. aureus > S epidermidis > S.haemolyticus > S.hominis.

