Placing endosteal implants in highly resorbed alveolar ridges is hardly possible due to the risk of mandibular nerve damaging or maxillary sinus floor perforation. The last one may lead to sinusitis and sinus fistula formation [1, 4]. By advanced bone resorption in posterior maxilla sinus augmentation is recommended – it increases the volume of the bone while lifting the sinus floor level. As any other treatment option, this one also has its indications and contraindications. In severely resorbed mandible osteoplasty or nerve repositioning can be carried out. However, as it is known from experience, ridge reconstruction by such a pathology is undesirable or impossible because of postoperative complications. High muscle attachments in severely resorbed mandible displace graft material during function. An often complication related to nerve repositioning is permanent disturbance of sensibility in the zone innervated by n. alveolaris inferior. This fact undermines the reasonability of this method of treatment.
In the above mentioned situations placing customized subperiosteal implants is viewed to be the best treatment option.
Experimental researches launched in the early 50s focused on studying tissue response to endosteal implants. Pasqualini U. was one of the first to conduct a series of experiments on dogs. He used implants made of acrylic resin, porcelain, gold and binary alloys. He discovered an earlier unknown bone response pattern – a close contact between the implant and the bone without any connective tissue interface, preserved as well after functional load apply.
Attempts to find the most bone-friendly material continued. The first symposium on alloplastic implants was held in Padua in 1955. It was there A. Hammer and G. Pallazi, relying on their own morphological investigations, advocated the absence of any negative response to implants made of Co-Cr alloy.
A. Bodine studied tissue response to a subperiosteal implant being in function for several years in dog maxilla. He reported the results of this morphological study at the conference in Dallas (USA) in 1956. His conclusion was: “The tissue in contact with implant parts under periosteum was a typical connective tissue.”
Exploring bone response to implants made of different alloys is one of keys to advance with materials for dental implants.
The aim of our project was to monitor and compare the dynamics of the integration process for different implants placed in rabbit mandibles (stainless steel, titanium alloy VT-01 and titanium alloy VT-01 with plasma-sprayed particulate titanium coating).
Following criteria were used in order to evaluate bone response to implantation:
– state of the original bone in the implant site
– dynamics of connective tissue interface-formation and -maturation
– bone-formation and maturation
– speed and completeness of original bone restructuring
– quality of implant integration
Materials and methods of research
Implantation, followed by morphological analysis of the samples was performed on 3 groups:
1 – stainless steel plates implanted in rabbit mandibles,
2 – titanium alloy VT-01 plates implanted in rabbit mandibles,
3 – titanium alloy VT-01 plates with plasma-sprayed particulate titanium coating implanted in rabbit mandibles.
Results of research and their discussion
Implantation of stainless steel plates
1-month results. Implants were removed before histological analysis. Therefore histological photos reveal vacuum instead of implants.
A thick layer of connective tissue was usually present at the interface between the bone and the implant (fig. 1).
Connective tissue interposed between implants and bone was recognized to be highly cellular loose connective tissue. Original bone subjected to moderate resorption. As a rule, bone trabeculae deposited on the original bone from outside had immature matrix of osteoid character (fig. 1).
3-months results. A layer of loose connective tissue was still present at the bone-to-implant interface, having this time more collagen fibers. It separated the implant from newly formed bone trabeculae, which had either osteoid or coarse matrix type (fig. 2). Collagen fibers predominated in this fiber-rich matrix; the latter was also highly cellular due to high content of fibroblasts together with polyblasts and macrophages (fig. 3).
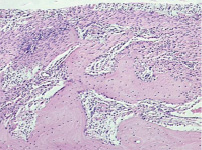
Fig. 1. Microphotograph X 200 Implantation of a stainless steel plate, 1-month results. A broad connective tissue interface between the bone (slight resorption) and the implant. Apposition of bone trabeculae onto the original bone
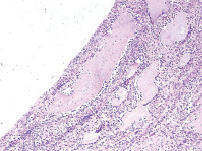
Fig. 2. Microphotograph X 100 Implantation of a stainless steel plate, 3-months results. A thin connective tissue interposed between newly formed trabeculae and the implant. The original bone has become compact. Huge osteoclasts can be quite often seen in the structure
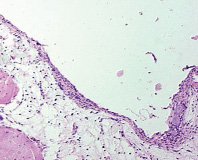
Fig. 3. Microphotograph X 200 Implantation of a stainless steel plate, 3-months results. A diffused lympho-macrophage infiltrate with some separate huge polynuclear cells in the connective tissue layer
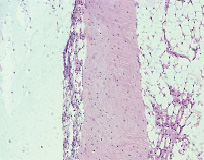
Fig. 4. Microphotograph X 100 Implantation of a stainless steel plate, 6-months results. A thin connective tissue film surrounded by loose connective tissue and original bone structures
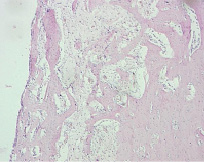
Fig. 5. Microphotograph X 50 Implantation of a titanium plate, 1-month results. A thin connective tissue layer surrounds the implant. Active apposition of new trabeculae onto the original bone
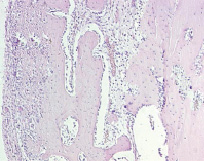
Fig. 6. Microphotograph X 100 Implantation of a titanium plate, 1-month results. High cellularity of a fairly broad connective tissue layer as a result of diffused infiltration with lymphocytes and macrophages. Active bone formation on the surface of the original bone. The latter is undergoing reactive rarefication
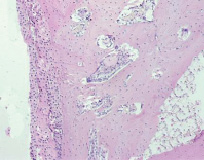
Fig. 7. Microphotograph X 100 Implantation of a titanium plate, 6-months results. Bone adjacent to the implant is becoming compact. It is separated from the implant only by a row of connective tissue cells
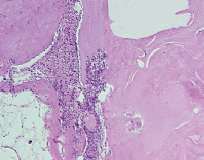
Fig. 8. Microphotograph X 200 Implantation of a titanium plate, 6-months results. The presence of separate thin fibrils and flat fibroblast-like cells is revealed
The original bone outside the forming bone structure tended to show rarefication. Active bone remodeling, followed by resorption of mature bone and new bone formation fostered pronounced bone “rejuvenation” in some parts.
Connective tissue interface was extremely thin in some areas. It was sometimes infiltrated with lymphocytes, macrophages and huge polynuclear cells.
On the whole, the newly formed bone was much better differentiated than the one in the 1 month-study. However, there were areas at the bone-to-implant interface with huge polynuclear osteoclasts. This fact, together with high cellularity of the tissue, testified to pathogenic influence of implant material.
6-months results. A thin film of connective tissue was found in the periimplant space. Under it one could see areas of loose connective tissue or structures of original bone (fig. 4).
Connective tissue adjacent to the implant had some loose areas infiltrated with short collagen bundles alternating with reticular fibers. An increase in small blood vessels could also be noted here and there.
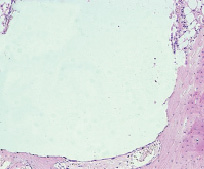
Fig. 9. Microphotograph X 50 Implantation of a titanium plate, 6-months results. Bone adjacent to the implant is becoming compact. It seems to have a direct contact with the implant in some parts
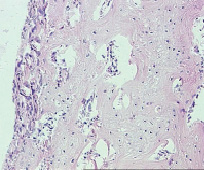
Fig. 10. Microphotograph X 100 Implantation of a titanium plate with plasma-sprayed particulate titanium coating, 1-month results. Implant is surrounded by a layer of highly cellular fibrocellular connective tissue. Active bone formation on the surface of the original bone
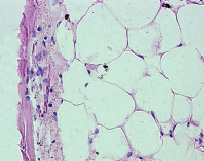
Fig. 11. Microphotograph X 400 Implantation of a titanium plate with plasma-sprayed particulate titanium coating, 3-months results. An area of adipose tissue in the original bone. It is separated from the implant by a thin fibrous layer. Osteoid formation in the latter (arrows)
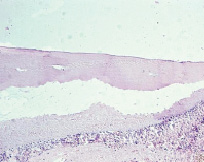
Fig. 12. Microphotograph X 100 Implantation of a titanium plate with plasma-sprayed particulate titanium coating, 6-month results. Full osteointegration. A barely noticeable notched form of the bone surface adjacent to the implant
Connective tissue interface from the implant side was vastly infiltrated with lymphocytes and macrophages including some single huge polynuclear cells. The original bone was in some parts dense and completely matured by that time.
Implantation of titanium alloy VT-01 plates
1-month results. A thin layer of connective tissue was present at the bone-to-implant interface. We observed intensive trabeculae formation on the surface of the original bone (fig. 5). High cellularity of the connective tissue layer owed to lympho-macrophage infiltration (fig. 6).
3-months results. A thin connective tissue film was still present at the bone-to-implant interface. We registered osteoid formation and homogeneous structure of intercellular matrix in several parts.
A broad net of newly formed trabeculae could be observed outside the connective tissue interface. Intertrabeculae spaces were filled with loose connective tissue rich in thin blood vessels. Huge polynuclear cells could be found near some of the trabeculae. The bone adjacent to the implant was undergoing the process of compactisation.
6-months results. The bone structure immediately adjacent to the implant was either porous or compact (fig. 7–9). The layer of connective tissue interposed between implants and bone was very thin limited sometimes to a bare row of connective tissue cells (fig. 7–9). It could even give an impression of a direct bone-to-implant contact without interposition of connective tissue. Detailed study of histological preparations revealed, however, the presence of separate thin fibrils and flat fibroblast-like cells.
Implantation of titanium alloy VT-01 plates with plasma-sprayed particulate titanium coating
1-month results. A highly cellular layer of fibrocellular connective tissue separated the bone from the implant. We observed intensive trabeculae formation on the surface of the original bone (fig. 10).
3-months results. A thin connective tissue film with plenty of collagen fibers bordered the implant. Intercellular matrix had sometimes homogeneous structure with apposition of osteoid (fig. 11). Thus, osteointegration could already be traced beginning from a 3-months period.
6-months results. The 6-months results indicate full osteointegration of the implants. Notched form of the bone adjacent to the implant witnessed high congruence of bone and implant surfaces (fig. 12).
Conclusions
The study showed that implantation of stainless steel plates into the rabbit mandibula led to formation of a connective tissue capsule around implants. 3- and then 6-months results demonstrated maturation of its fibril network and its collagenisation. Periimplant regions experienced chronic inflammatory process that impeded tissue maturation in close contact to implants, as well as osseointegration. The last statement can also be confirmed by the presence of a connective tissue interface during the whole period of experiment.
As for implantation of titanium plates (groups 2 and 3), we came to the conclusion that titanium possesses high biocompatibility. In contrast to group 1, where implantation of stainless steel plates caused prolonged inflammation in a 1–6 months period, here we saw rapid maturation of fibrous capsule around implants.
Furthermore, the periimplant area in groups 2 and 3 clearly indicated active osteogenesis with more rapid maturation of bone structure during remodeling. The contact of titanium implants with the bone brought to fibroosseous integration. It should be noted that the process of osteogenesis was much more active in group 2 compared to group 1. 6 months after implantation the contact with the bone was so close (group 2) that it imitated full osteointegration. Only a hardly visible row of fibroblast-like cells evidenced the involvement of connective tissue into the process.
We suppose that osteointegration was most active in group 3. Areas of direct bone-to-implant contact could already be observed at 3 months following implantation. 6 months after implantation they became prevalent – the fact which validates osteointegration.

