3-aryl (hetaryl) hydrazono-3H-furan-2-ones constitute an important class of heterocyclic compounds, a promising object for further research in the field of heterocyclic chemistry [1]. Their structure contains several reaction centers, allowing them to enter into both electrophilic and nucleophilic reactions; introduction of various substituents into their hydrazone fragment or heterocycle increases their reaction potential [2].
The synthesis of these compounds is based on the use of unsubstituted 5-aryl-3H-furan-2-ones in coupling reactions with diazonium salts. 3-Aryl (hetaryl) hydrazono-3H-furan-2-ones are proven to exist in the E configuration and in the hydrazone form, which is confirmed by X-ray diffraction data [3, 4]. The existence of compounds 1 a-b in the hydrazone form allows one to suggest the course of alkylation and acylation reactions by the N-type.
The behavior of 3-arylhydrazono-3H-furan-2-ones 1 a, b under the action of bromoethane, propyl bromide, and benzyl chloride was studied. Alkylation was conducted by heating in the presence of a catalytic amount of potassium carbonate in acetone solution for 8 h. Under similar conditions, acylation reactions proceed by the action of benzoyl chloride.
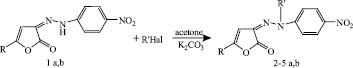
1a,b: R=Ph, Tol; 2 a,b: R=Ph, Tol; R’= 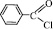 ; 3 a,b: R=Ph, Tol. R’= Et;
; 3 a,b: R=Ph, Tol. R’= Et;
4 a,b: R=Ph, Tol. R’= n-C3H7; 5 a,b: R= Ph, Tol. R’= 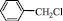 .
.
Examination of the spectral data of the compounds obtained confirms the reaction proceeding by the NH-moiety without affecting other possible centers. The IR spectra of the compounds identified as 5-aryl-3-(2-benzoyl-2-(4-nitrophenyl) hydrazono)-3H-furan-2-ones (2 ab) contain three intense absorption bands within 1,800–1,500 cm-1: for the C=O bond of the furanone cycle (1,800–1,750 cm-1), for the C=O bond of the benzoyl fragment (1,640–1,600 cm-1), and for the C=N bond (1,690–1,675 cm-1); any average-intensity bands within 3,300–3,100 cm-1 corresponding to the stretching vibrations of the NH bond of a hydrazone group are absent.
The IR spectra of the compounds identified as 5-aryl-3-(2-ethyl-2-(4-nitrophenyl) hydrazono)-3H-furan-2-ones (3 a, b) also lack the absorption band of a NH group (3,300–3,100 cm-1) but contain the absorption band of the C=O group (1,800– 1,750 cm-1). The 1H NMR spectrum shows a series of signals characteristic of the ethyl group protons: 2.79–2.84 ppm (2H q), 0.85–0.88 ppm (3H t); and no 1H singlet of a hydrazone group within 11.00–12.80 ppm.
The 1H NMR spectra of the compounds identified as 5-aryl-3-(2-propyl-2-(4-nitrophenyl) hydrazono)-3H-furan-2-ones (4 a-b) contain a series of signals corresponding to the propyl group protons: 3.55–3.59 ppm (2H t), 1.75–1.45 (2H m), and 0.85–0.88 ppm (3H t).
The 1H NMR spectra of the compounds identified as 5-aryl-3-(2-benzyl-2-(4-nitrophenyl) hydrazono)-3H-furan-2-ones (5 ab) show a 2H singlet of the CH2 group in the benzyl radical around 4.35 ppm, as well as a multiplet of aromatic protons within 7.32–8.26 ppm, but no 1H singlet of a hydrazone group.
This work was supported by the Russian Foundation for Basic Research (project № 16–03–00530a).

