Recently the usage of transparent conductive film (TCO) is growing rapidly. The most popular transparent and conductive oxide is indium tin oxide In9SnO15-x (ITO). ITO films have a high light transmittance in the visible spectrum, good electrical conductivity, hardness and chemical inertness. They can be widely used in the construction and automotive industry, provided that they obtain a low-cost large-scale method. ITO film reflects infrared rays and they can be used it as a thermal barrier coating on the window panes [1].
The application of TCO films as transparent electrodes for electrochromic glass is actual. Electrochromic glasses are used in the buildings and window and offices dressing, automobile industry for the auto-dimming rear-view mirror of the car. Electrochromic glass reduces the heat loss and costs of air conditioning and lighting. They are an alternative to mechanical shutters and shading screens or curtains. Absorption or reflection of light in the visible and near infrared region is regulated by the electric field applied. Dynamic control of sunlight and infrared radiation can significantly reduce energy consumption in hot summer and cold winter conditions. But now the electrochromic glass has a relatively high cost.
To reduce the cost of the electrochromic glass need to develop the low-cost technology of their production. Availability of raw materials and the cost of manufacturing method are important factors in the manufacture of functional materials. The choice of method is generally associated with optimal operation considering of thin solid film for a particular use and minimizing manufacturing costs.
Oxide films traditionally deposited by vacuum sputtering of targets heated to high temperatures [2]. Now developed larger machines with complex processes of measurement and control systems to stabilize the reactive sputtering process. The method of laser sputtering [3] allows to spray almost any composition of the target. Ionic plating method [4] uses a fully automated vacuum chamber with a cryo-pump equipped with analytical devices. Sol-gel method [5] can be used to produce quality films with a wide possibility of changing properties with changing the solution composition.
Physical and chemical properties of the resulting film (resistivity, optical transmittance, surface roughness) correspond to the method of deposition and process conditions. Properties of oxide films is determined by technological factors which ensuring the homogeneity of the material and stoichiometry. In particular, the extraction-pyrolytic method provides the mixing of components in solution in a given proportion and maintaining the stoichiometry of obtaining materials.
This paper describe the transparent conductive films and electrochromic glass obtained by extraction-pyrolytic method [6]. Extracts of metals are characterized by low content of impurity elements at the level of 5.10 mg / l and the adjusted concentration unchanged during storage.
Materials and methods of research
In this paper, for the synthesis of transparent conductive oxide InSnO films and electrochromic NiO films, the extraction-pyrolytic (EP) method [6] has been used. The method consists of the extraction of metals from solutions of inorganic salts for purification from impurities and transfer of the metal to organic phase. The obtained extracts – salts of organic acids – wet the substrate well of any type and form of self-assembled thin film. Metal concentrations in the extracts were estimated by the atomic absorption technique with the AAS-1M device. The organic extracts were mixed in the required stoichiometric ratios and diluted to a predetermined concentration, the most optimal for the formation of thin films.
The films were deposited by rolling of extract on a glass substrate which has been previously cleared. The organic extracts well moistened the glass substrate and form a self-assembled thin film. After drying the wetting film are placed in a open pyrolysis furnace. After drying the substrate, the wetting film was placed in a furnace for pyrolysis in air at a temperature of 450ºC. Pyrolysis of wetting film results the formation of many nucleation centers and result the nanostructured complex oxide films. It is found that the solution density 0.93 g/cm3 provides after pyrolysis a continuous conductive film which thickness about 30 nm.
The images of films were investigated by atomic force microscopy (AFM) using a multimode scanning probe microscope (Veeco, USA). The electrochemical device was assembled and tested with the application of direct and reverse current through the DC generator (DAZHENG-Electronics, Model: PS-305D). The measurements of transmittance films were performed on a Fourier spectrophotometer (VERTEX-80V, Bruker Corp.) in the wavelength range of visible light and mid-infrared (IR) range.
The potentiodynamic curves were displayed on a potentiostat PI-50-1. The sweep rate was 10 mV/s.
Results of research and their discussion
To form the electrochromic device the nickel oxide film is formed on the surface of the ITO-electrode from solutions with different concentrations of nickel extracts. The obtained NiO film had a dark color, which is intensified with increasing film thickness. Transmittance of NiO film with thickness of 150 nm (5 layers) was 62 %, for NiO film with thickness of 300 nm (10 layers) – 51 %, and for NiO film thickness of 450 nm (15 layers) – 41 %. Proceeding from these data, you can choose the required degree of staining of the electrochromic glass, taking into account the degree of discoloration of the film upon application of an electric current. As shown by further studies, the discoloration of NiO film slightly increases with decreasing its thickness (from 85 to 81 %).
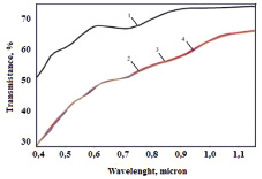
Fig. 1. Transmission spectrum of EC cell produced by EP method (visible range). 1 – bleached state cycle number: 1; 2 – coloured state cycle number: 1; 3 – coloured state cycle number: 100; 4 – coloured state cycle number: 500
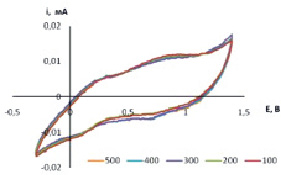
Fig. 2. Potentiodynamic curves of a two-electrode cell at a scan rate of 10 mV/s. Extents of the cycles are shown in the graph. Electrolyte: 1 M propylene carbonate solution of LiClO4 + PEG
The obtained NiO films had a dark colour, which intensified with increasing film thickness. In order to study the possibility of using NiO film in electrochromic devices, the dependence of light transmittance at wavelength λ = 633 nm on the thickness of NiO films was investigated. Transmittance spectra of EC cells in the visible and IR regions at different work cycles are illustrated in Fig. 1.
Spectra of cell transmission in the bleached and colored states to 1 and 500 cycles are coincided. The transmittance of light in the visible range was 70–80 % without voltage application. ITO film on the glass absorbs the UV light. The transmittance in the IR region is averaged 10–40 % in the colored state and 10–55 % in the bleached state. There is not transmission in the far infrared.
The applied voltage (approximately 12 V) with an appropriate polarity introduced a charge into the electrochromic material that causes a change in absorption in the visible region of spectrum. The potentiodynamic curves are shown in Fig. 2.
According to the data of potentiometric cycling of electrochromic cells during the first 500 cycles passes with Coulomb efficiency greater than 98 %, with comparable to the cathode and anode current, and thus charge and discharge capacity. That, indicate to the complete reversibility of the processes. The color change can be attributed to nickel oxide transition from one phase to another. Nickel oxides are non-stoichiometric. Nonstoichiometry is accompanied by a change of color from green to black due to the Ni (III)existence.
The developed method can be used for deposition of functional coatings on large surfaces, such as the transparent surface and cabinet doors and windows. Such coatings help to save energy and improve the environment.
Conclusion
The electrochromic device in which nickel oxide film transfers from the reduced NiOOH state to the oxidized Ni(OH)2 state with change from the colour brown to transparent has been produced by extraction-pyrolysis technique. Optimally, for obtaining a uniform electrochromic NiO film, the extract solution with concentration of 2 % should be used. According to the thermogravimetric data, the oxide film formation occurs at 370–450 °C. The resulting NiO film on transparent conductive glass, combined with the counter-electrode in the presence of quasi-solid electrolyte, withstood more than 500 cycles of discolouration–staining without changing the intensity of the colour.

