Interest to the arylmethylidene derivatives of 3H-pyrrol-2-ones is due to a wide spectrum of their useful properties, many of which find application in medicine, industry, and agriculture, their analogues are parts of alkaloids and drugs. Of special interest is modification of the pyrrolonic cycle under the action of nucleophilic reagents. We studied the reaction of 3-arylmethylidene-3H-furan-2-ones with binucleophilic - 2-mercaptobenzymidazole. The interaction under study can proceed by several reaction centers, which enables the substrate´s structure to be modified by means of regioselective chemical transformations.
5-R-3-arylmethylidene-3H-furan-2-ones are known to react with 2-mercaptobenzymidazole at heating, in a solution of icy acetic acid, in the presence of catalytic amounts of concentrated sulfuric acid and do not interact at boiling in an ethanol solution, in the presence of catalytic amounts of triethylamine (potassium carbonate, sodium alcoholate) [1].
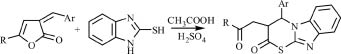
We studied the interaction of 5-R-3-(2-chlorobenzylidene)-3H-pyrrol-2-ones Ia,b and 5-phenyl-3-(4-methoxybenzylidene)-3H-pyrrol-2-one Ic with 2-mercaptobenzymidazole at heating during 30 hours in a solution of icy acetic acid and catalytic amounts of sulfuric acid. In the structure of the arylmethylidene derivatives of pyrrol-2-ones there are several reaction centers liable to nucleophilic attack, namely, an exocyclic double C-C bond, a carbonylic group, labile bonds in the cycle (C-N and C-H); besides, the presence of a second nucleophilic center in the reagent promotes its attack by the carbonylic carbon atom and closing the corresponding five-membered or six-membered cycles.
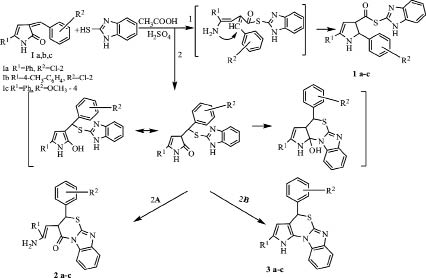
The data of elementary analysis and spectral characteristics, in combination with quantum mechanical computations, suggest the compounds formed to be 3-(2-amino-2-R1-vinyl)-2-R2-2H-benzo[4,5]imidazo[2,1-b][1,3]thiazine-4(3H)-ones 2a-c, whilst the possibility of forming products 1a-c and 3a-c is excluded.
Reaction products 2a-c were isolated with a yield up to 73 %. The IR spectra of compounds 2a-c have the absorption bands of a carbonylic group 1698-1707 cm-1, NH2 3183-3201, 3426-3520 cm-1, vibrations of aromatic rings 1600-1620 cm-1. In the 1H NMR spectra of compounds 2a-c we note the signals of a vinyl proton within 5,68-5,71 ppm, the signals of the protons at tertiary carbon atoms within 5,72-5,74 and 6,82-6,92 ppm, the signal of a NH2 group´s protons at 9,97-9,99 ppm, the multiplet of aromatic rings within 7,17-7,69 ppm, the signal of the methyl group protons of an aromatic substituent (for compound 2b) around 2,35 ppm, and that of a methoxylic group (for compound 2c) at 3,82 ppm.
We also tried to implement reaction of 5-R1-3-R2-3H-pyrrol-2-ones with 2-mercaptobenzymidazole under microwave radiation. Only the source compounds were detected in the reaction mixture, which means this reaction not proceeding under microwave radiation.
Experimental
IR spectra were recorded on an FSM-1201 Fourier spectrometer in KBr tablets, the spectral range being 400-4000 cm-1. 1H NMR spectra were obtained on a Varian-400 spectrometer within 20-25 °C in CDCl3, TMS being the internal reference. The working frequency was 400 MHz.
3-(2-amino-2-R1-vinyl)-2-R2-2H-benzo[4,5]imidazo[2,1-b][1,3]thiazine-4(3H)-ones (2a-c). A mixture of 5-R-3-arylmethylidene-3H-furan-2-one (Ia, b, c) (0,01 mol) and 2-mercaptobenzymidazole (0,015 mol) was boiled in icy acetic acid with a catalytic amount of sulfuric acid during 30 hours, poured into cold water, and neutralized by a sodium carbonate solution. The crystals precipitated were filtered on a Schott filter and recrystallized from ethanol.
For 2а: yield 73 %; mp 263-265 °C; 1H NMR, δ: 5,70-5,71 (1Н, d), 5,72-5,73 (1Н,d), 6,86-6,90 (1Н, t), 9,97 (2Н, NH2), 7,19-7,66 (13Н, m, Ar). Found (%) C, 66,74; H, 3,94; N, 6,69; S, 7,65. Calc. for C24H18N3SOCl (%) C, 66,74; H, 4,17; N, 7,73; S, 7,42.
For 2b: yield 71 %; mp 249-251 °C; 1H NMR, δ: 5,70-5,71 (1Н, d), 5,73-5,74 (1Н,d), 6,89-6,92 (1Н, t), 9,97 (2Н, NH2), 7,17-7,63 (13Н, m, Ar), 2,23 (3H, s). Found (%) C, 67,92; H, 4,60; N, 4,05; S, 7,50 Calc. for C25H20N3SOCl (%) C, 67,33; H, 4,52; N, 9,42; S, 7,19
For 2с: yield 68 %; mp 141-142 °C; 1H NMR, δ: 5,68-5,69 (1Н, d), 5,72-5,73 (1Н,d), 6,82-6,86 (1Н, t), 9,99 (2Н, NH2), 7,20-7,69 (13Н, m, Ar), 3,82 (3H, s). Found (%) C, 68,75; H, 4,33; N, 8,10; S, 7,44. Calc. for C25H21O2N3S (%) C, 69,40; H, 4,95; N, 9,83; S, 7,50.
References
1. Anis´kova T.V., Yegorova A.Yu., Chadina V.V. The synthetic capabilities of 3-arylme-thelene-3H-furan-2-ones in the reaction with N,-C-,S-nucleophilic reagents // 5th International Conference on Organic Chemistry for Young Scientists (Abstract of Reports.), 2009. - Saint-Petersburg: 2009. - P. 92.
The work is submitted to the Correspondence electronic conference «Biologically active connections reception, properties, structure, functions, application». The work was supported by Russian Foundation for Basic Research (grant № 10-03-00640-a).

