Diagnostics and treatment of colonic polyps and polyposis are the issue of today due to steady growth of the number of new cases. Most of researchers adhere to the opinion, that polyps are precancerous condition and tumors go through long stage of adenomatous polyp and 95% of malignant tumors develop from adenomatous polyps, and their removal is the way to prevent cancer [3, 9, 10, 13, 15, 19]. At the same time, regardless to surgical and conservative treatment the recurrences of the disease, complications leading to severe suffering of patients and hesitance in curability of it are quite frequent [1, 4, 11, 18, 20, 25, 28]. This is related to different factors, amongst which the genetic predisposition to development of colonic polyps and polyposis (CPP) and colonic cancer is believed to be one of the most important [5, 6, 8, 14, 26, 27].
In the context of the modern diagnostics of colonic polyposis, prognostication of malignization of polyps and choice of adequate surgical tactics, and issues of complex diagnostics of the disease on different levels of health care system, including rational use of modern diagnostic methods are important issues.
Objective
Development of diagnostic and prognostic criteria of severity of the disease and the risk of malignization of colonic polyps for health care institutions of different levels, using modern diagnostic methods (virtual colonoscopy, methods of molecular genetics).
Materials and methods
There are analyzed the diagnostic and treatment outcomes of 183 patients with colonic polyps and polyposis treated at the Republican Coloproctology Research Center (RCPRC) and Republican Oncology Research Center (RORC) of the Ministry of Health of Uzbekistan in 1998-2008.
The diagnosis of colonic polyps and polyposis has been made based on the outcomes of clinical-instrumental and laboratory tests based on the classification of V.D. Fedorov, 1983.
Forms and stages of the diffuse colonic polyposis were differentiated in line with recommendations of V.P.Rivkin, 2006 and based on morphological analysis of biopsy specimens of colonic mucosa.
For diagnostic purposes all examined patients undergone complex clinical-instrumental examination, which included endoscopy, X-ray examination, including virtual colonoscopy (VC) multispiral computed tomography (MSCT) using the device of PHILIPS. There were also performed clinical blood and urine tests, as well as biochemical blood assay.
Genetic part of the survey has been done in 64 patients with different forms of polyps and polyposis and in 20 healthy volunteers with unburdened genetic background.
Genetic research has been done in collaboration with the Laboratory of Human Functional Genomics at the Institute of Genetics and Embryology of Uzbek Academy of Sciences. DNA from the tissue specimens has been isolated using Wizard Genomic DNA Purification Kit (Promega, США) following the producer instructions. For PCR-amplification of fragments of the genes being analyzed the appropriate primers have been used.
Results
The age of patients ranged from 14 до 78. There were 111 (60.7%) males and 72 (39.3%) females (Table 1). Ratio of males and females made 1.54:1.
Table 1
Distribution of patients by sex and age, n=183
|
Age of patients, years |
Males |
Females |
Total |
|||
|
abs. |
% |
abs. |
% |
abs. |
% |
|
|
Under 20 |
5 |
2,7 |
4 |
2,2 |
9 |
4,9 |
|
20-29 |
26 |
14,2 |
20 |
11,0 |
46 |
25,2 |
|
30-39 |
15 |
8,2 |
4 |
2,2 |
19 |
10,4 |
|
40-49 |
21 |
11,5 |
21 |
11,5 |
42 |
23,0 |
|
50-59 |
24 |
13,1 |
20 |
11,0 |
44 |
24,1 |
|
60 and older |
20 |
11,0 |
3 |
1,5 |
23 |
12,5 |
|
Total |
111 |
60,7 |
72 |
39,3 |
183 |
100,0 |
|
Average age |
43,7±1,45 |
39,7±1,56 |
42,2±1,08 |
|||
Duration of disease varied in wide range. Duration from 1 month to 1 year was in 36 (16,7±2,9%) patients, from 1 to 2 years - in 65 (35,5±3,5%), from 2 to 3 years - in 32 (17,5±2,8%), more than 5 years - in 34 (18,6±2,9%) patients. Duration of the disease up to 1 year was mainly in patients with solitary polyps and juvenile form of diffuse polyposis, from 1 to 2 years - in patients with hyperplastic form, 2-5 years - with adenomatous and adenopapillomatous form.
Solitary polyps are found in 52 (28,4±3,3%), multiples - in 45 (24,6±3,2%), diffuse colonic polyposis - in 71 (38,8±3,6%) patients, out of them Peuta-Jeghers syndrome was found in 13 (7,1±1,9%); in 15 (8,2±2,0) patients malignant polyposis of colon has been found.
By the level of dissemination of polyps the patients are distributed as follows: distal lesions in 135 (73,8±3,3%), left part of colon in 18 (9,8±2,2%), subtotal in 7 (3,8±1,4%), total in 23 (12,6±2,5%) patients. Mild cases were found in 62 (33,9±3,5%) patients, moderate - in 78 (42,6±3,7%), severe - in 43 (23,5±3,1%).
Distribution of patients by forms and stages of diffuse polyposis of colon: proliferating diffuse polyposis has been found in 35 (19,1±2,9%) patients, out of them I (hyperplastic) stage - in 25 (13,7±2,5%) patients, adenomatous - in 78 (42,6±3,7%), adenopapillomatous polyposis - in 54 (29,5±3,4%). According to publications data while making biopsy it is important to pay attention to the technique of sampling and histological sections preparation [1, 7, 15, 18, 21, 31]. While biopsy sampling during colonofiberscopy, it is important to make electroscission at polyps peduncle with minimal traumatizing of its rest parts. While taking a sample the excisions has to include the end, peduncle and base of the polyp. The main clinical features of polyposis included symptoms of intoxication, extraintestinal and gastric manifestations (Table 2). The most frequent symptoms were: melena (65,6±3,5%), general weakness (59,6±3,6%), abdominal pains (28,4±3,3%), weight loss (24,6±3,2%), anal pains (16,4±2,7%).
Table 2
Main clinical manifestations of colonic polyps and polyposis, n=183
|
Claims |
Number of patients |
|
|
абс. |
% |
|
|
Intestinal manifestations: - melena - abdominal pains - bleeding - presence of pus and mucus - tenesmus - meteorism - diarrhea - constipation |
120 52 67 16 11 8 2 2 |
65,6±3,5 28,4±3,3 36,6±3,6 8,7±2,1 6,0±1,8 4,4±1,5 1,1±0,8 1,1±0,8 |
|
Intoxication symptoms: - general weakness - weight loss - dizziness - dry mouth |
109 45 17 2 |
59,6±3,6 24,6±3,2 9,3±2,1 1,1±0,8 |
|
Extraintestinal manifestations: - anal pains - prolapse of polyps during defecation act - availability of formation in anal part - liquid stools and gas incontinence |
30 9 4 3 |
16,4±2,7 4,9±1,6 2,2±1,1 1,6±0,9 |
|
Gastric manifestations: - appetite loss - nausea - vomiting |
8 2 2 |
4,4±1,5 1,1±0,8 1,1±0,8 |
Table 3
Complications of the underlying disease in patients with colonic polyps and polyposis, n =183
|
Complications |
Number of patients |
|
|
Abs. |
% |
|
|
Bleeding |
67 |
36.6±3.6 |
|
Hemorrhagic anemia |
53 |
29.0±3.4 |
|
Abdominal pains symdrom |
51 |
27.9±3.3 |
|
Chronic colonic obstruction |
18 |
9.8±2.2 |
|
Malignization |
15 |
8.2±2.0 |
|
Cachexy |
9 |
49±1.6 |
|
Strictures |
9 |
4.9±1.6 |
|
Acute colonic obstruction |
8 |
4.4±1.5 |
|
Pericolic abscesses |
5 |
2.7±1.2 |
|
Perforations |
2 |
1.1±0.5 |
|
Paraproctitis |
1 |
0.5±0.5 |
Amongst the complications the most frequent ones were hemorrhages (36.6±3.6%), hemorrhagic anemia (29,0±3,4%), abdominal pain syndrome (27.9±3.3%) (Table 3).
Molecular-genetic research helped to find out, that the diffuse colonic polyposis syndrome is caused by germinal mutation of the suppressor gene of the tumor - APC (Adenomatous polyposis coli). Besides, APC has the oncogene function, because some mutant forms of APC not only lose their normal function, but become able to fix and inactivate normal APC protein. Occurrence of the somatic mutation in a normal allele leads to inactivation of both alleles and occurrence of sporadic colorectal cancer cases [5, 6, 8, 14, 16].
Out of 64 genetically tested patients mutation in APC gene has been found in 51 (79.7±5.0%), in such a case, the frequency of its occurrence depended on the form of the disease. In 5 patients there were solitary, in 15 - multiple and in 44 - diffuse polyps, and out of the latter the Peuts-Jeghers syndrome was found in 11. In 16 patients there were found distal, in 8 - left side, in 6 - subtotal and in 34 - total affection of colon. Status of 10 patients was qualified as light, 20 patients - moderate, and in 34 patients as severe. 27 patients have undergone different operations on the occasion of multiple polyps and polyposis. In 27 patients there were found concomitant diseases (cardiovascular, lung, liver, gastro-intestinal tract and endocrine system). In 9 patients there was revealed malignant polyposis.
Availability of mutations of АРС gene made significant influence on the course of the pathologic process. Thus, there were no mutations of APC gene found in patients with light course of disease. In patients with moderate and severe course the frequency of mutations made 85.0±8.0% and 100.0±0.0% accordingly. There is also revealed the connection between the frequency of mutations and the extent of the pathologic process: in cases with distal and left side spread of polyps the frequency of mutations was 56.3±12.4 и 75.0±15.3%, whereas in cases of subtotal and total affection of colon - mutations were found in all examined patients (100.0±0.0%). Also if in absence of mutations there were mainly affected distal parts of colon, polyps were of small size and no complications of the underlying disease were found, in patients with mutations there was found subtotal and total affection of colon, with polyps in shape of "bunch of grape", big size polyps on the flat base. In patients with mutations in APC gene, especially those with Peuts-Jeghers syndrome, there was found total affection of colon with development of constrictions, malignization, polyps in shape of "bunch of grape". Quite frequently there was noted cachexy, posthaemorrhagic anemia. All the abovementioned indicate that molecular-genetic testing has to be included to the compulsory diagnostic complex of testing in cases of colic polyps and polyposis. It will allow to improve the results of surgical treatment, facilitating choice of adequate operative tactics. In patients with mutations in APC gene (mainly in cases of Peuts-Jeghers syndrome) there was also found affection of upper parts of gastro-intestinal tract and gallbladder with polyps.
According to results of ultrasound scanning in 2 out of 11 patients with Peuts-Jeghers syndrome there were found polyps of gallbladder (Picture).
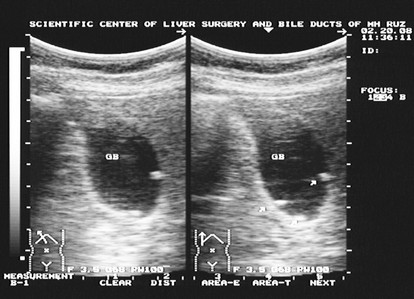
During colonofiberscopy and irrigography in patients without mutations in he gene there were found mainly lesions of distal parts of colon, while in patients with mutations in the APC gene there were found the signs of toxic dilatation, stricture, filling defects and "bovine eye" syndrome.
Thus, in patients with multiple and diffuse polyposis, especially those with family polyposis and Peuts-Jeghers syndrome the APC gene mutations are found with high frequency (up to 100%) There is definite connection between availability of mutations in APC gene and inherited predisposition, course of the pathologic process, its extent of spread, clinical manifestations and complications.
Discussion of the results and justification of the complex diagnostic´s algorithm
The main screening principles are based on 3 principles: while making decision on the method and time of starting the screening the family and individual risk factors have to be evaluated; physician has to recommend the further examination in case of positive results of screening; patient has to be informed about both positive and negative sides of each test, in order to make informed choice.
One of the most accessible screening methods is occult blood feces analysis. However, according to literature [2, 3, 4, 15, 16, 19], its sensitivity in patients with colic polyps and polyposis does not exceed 50%. According to our data it is between 0 and 17.6%, depending on the size of polyps: 0-1% in polyps up to 1 mm, 8-17% - in polyps from1 to 7 mm, and 17.6% - when the diameter of polyp is more than 8 mm. Besides the specificity of this method, according to our data, is 7.2% in polyps with diameter of 1-7 mm, and 11.3% in cases when diameter iss 8 mm and bigger. The diagnostic accuracy is 1% in diameter of the polyp up to 1 mm; 3% if diameter is 1-7 mm, and 9% if diameter is 8 mm and higher.
The other widely used method - sigmoidoscopy, exceeds in its sensitivity the previous one. In polyp sizes up to 1 mm, 1-7 mm, 8 mm and more it constitutes accordingly 3-8, 18-31 and 58,2%, specificity of this method is - 2,1; 22,4 and 61,5%, and diagnostic value - 4, 18 and 31%. From the other side sigmoidoscopy in cases of big polyps and strictures does not allow examining the small intestine and colon fully, besides it is quite painful procedure, which frightens patients.
Colonofiberoscopy is the third by its information value instrumental method. [12, 15, 20, 24, 28, 29]. According to our data sensitivity of this method makes 28-42, 60-70 и 87,5%, specificity - 58,3; 64,5 и 90,1%, and diagnostic preciseness - 53,6; 70,3 и 80,7%, in cases of size of polyps up to 1 mm, 1-7 mm, 8 mm and higher - accordingly. The method of course requires thorough preparation of the patient, full cleaning of colon. Quite often there are noted false-positive results and impossibility of morphological diagnostics. One can speak about successful colonofiberscopy only in case of reaching by the device to head of blind colon. However if there is a stricture and colon deformation, the diagnostic possibilities and value of the method are significantly reduced. At the same time colonofiberscopy does not provide information about the internal structure of polypoid formations and does allow identifying the depth of invasion of the malignant tumor to the colic wall, its invasion to neighboring organs, as well as about the condition of regional lymphatic nodes.
Since 1994 for diagnostics of colonic lesions there is successfully used non-invasive method VC, based on analysis of multiple sections, obtained using CT scan [22, 23, 27, 30, 31, 33, 32, 34, 35, 36]. Our results have shown, that in cases with polyps size less than 1, 1-7 mm, 8 mm and more the sensitivity of VC makes respectively 75-80, 100 and 100%, specificity - 85,1; 98,6 and 100%, diagnostic accuracy - 95,3; 100 and 100%. At the same time, this method cannot allow to receive biopsy material from lesion focus and make morphological investigation.
While developing the algorithm of the complex diagnostic of polyps and polyposis we proceeded from the following principal requirements:
1. All levels of the health care system have to be involved to the complex diagnostic process - primary health care (GPs at rural doctor´s stations and city family polyclinics), secondary care facilities (central rural and town polyclinics and hospitals), tertiary care (regional and republican specialized coloproctology centers).
2. Available diagnostic methods have to be applied taking into account both the level and possibilities at health care facilities, as well as the individual patient´s condition.
3. Complex diagnostics has to help to divide patients into groups, according to severity and spreading of the process, which identify the tactics of surgical and rehabilitation treatment. In this aspect the identification and forecasting of malignization of polyps is crucial.
Based on the results of our long-term observation and analysis of global experience, as well as taking into account the abovementioned requirements, we divided the patients into 4 groups, based on the disease severity and colic polyp´s malignization risk levels. We developed the complex of diagnostic and prognostic criteria for evaluation of severity of disease and malignization risk for different level health care facilities (Table 4).
Table 4
Complex of diagnostic and prognostic criteria to identify the severity of disease
and malignization risk of colic polyps
|
Clinical criteria |
Number and sizes |
Histology |
Diagnostic methods |
|
1 group - minimal risk of malignization |
|||
|
Clinical signs: blood in feces, anemia, possibly tenesmus and dropping out polyps during defecation act, family anamnesis, general symptoms (anemia, weight loss, abdominal pain, anal pain etc.) |
Solitary polyps with size no more than 5-8 mm, |
Adenomatous and villous polyps are prevailing |
Primary health care facilities: «hemoccult test», |
|
Secondary health care facilities (surgical depatrments): + oesophagogastrodudenofiberoscopy, anoscopy, rectoscopy, biopsy |
|||
|
2 group - moderate risk of malignization |
|||
|
Pathologic discharge and frequent liquid bloody stools, in combination with abdominal pain and meteorism, post hemorrhagic anemia, possibly tenesmus and drop-outs of polyps during defecation act |
Multiple polyps with size no bigger than 15 мм, up to 50-100 units, more frequently rectum and distal part of sigmoid colon is involved |
Proliferation, hyperplastic stage of polyposis |
Secondary and tertiary health care facilities (proctology units): Oesophagogastroduodenofiberoscopy, colonofiberoscopy, biopsy, preferably virtual colonoscopy |
|
3 group - significant risk of malignization |
|||
|
Pathologic discharges, frequent fluid bloody stools, combined with abdominal pain, meteorism, post hemorrhagic anemia, and cachexia |
Diffuse polyposis, initial stages of Peuts-Jeghers, Trucot, Gardner syndromes, polyps no bigger than 15-30 mm, 100-500 units, affection of rectum and colon |
Adenopapillomatous stage of diffuse polyposis with proliferation and dysplasia foci in epithelium of polyps with various manifestation degrees |
Republican coloproctology and oncology centers: oesophagogastroduodenofiberoscopy, colonofiberoscopy, biopsy, virtual colonoscopy, preferably molecular-genetic research (APC, PCR) |
|
4 group - high risk of malignization |
|||
|
Young age, family predisposition, presence of pigment and lentiginosis spots on the red border and hand fingers, enteric manifestations, anemia and cachexy |
Total diffuse colic polyposis, intestinal polyposis (Peuts-Jeghers, Trucot, Gardner syndromes) |
Peuts-Jeghers polyps with malignization or transformation of carcinoma in situ into adenocarcinoma within mucosa and myenteron |
Republican proctology and oncology departments and centers, oesophagogastroduodenofiberoscopy, colonofiberoscopy, biopsy, virtual colonoscopy, oncomarkers: carcinoempryonal antigen, molecular-genetic tests (АРС, PCR) |
While developing the complex we identified at first the 4 risk groups for malignization - minimal, moderate, significant and high, based on the need to apply different surgical tactics in each of them. There were developed main clinical criteria for identification with special focus on number and size of polyps, as well as their minute structure.
For each risk group there were identified diagnostic methods, relevant to the specific health care facility to ensure continuity in the process of examination of patients on all levels - from primary up to tertiary one. It facilitates timely send patients to the relevant facility - starting from family doctors and ending by the level of specialized proctology and oncology centers. Special emphasis has been done in the groups of moderate and especially high risk groups for use of the most up-to-date methods - virtual colonoscopy (VC) and PCR. VC, being the most high-informative and valuable method in diagnostics of polyps and polyposis, at the same time is still quite expensive and does not provide full answer in terms of extent or risk of malignization. Complementing it with PCR allows to assess the degree of malignization and, accordingly, to have more differential approach to the choice of surgical treatment tactics.
Conclusions
1. Early diagnostics and effective treatment of patients with colic polyps and polyposis requires improvement of screening and diagnostics system on all levels of health care system - starting from GPs and ending by coloproctologist and oncologists working at republican specialized centers - through identification of risk groups on the basis of both severity and malignization risk criteria.
2. In order to make efficient choice of the best surgical tactics in patients with colic polyps and polyposis, the complex of diagnostic examination of these patients has to contain modern high-informative non-invasive diagnostic methods, including the ones of molecular genetics for forecasting malignization processes, as well as virtual colonoscopy for assessment of extent and character of a lesion.
3. The developed complex of diagnostic and prognostic criteria of disease severity and risks of malignization of colic polyps meets all necessary requirements of early and effective diagnostics, and can be recommended for wide implementation in health care facilities of all levels.
References
- Abdullakhodjaeva M.S. Modern approaches in research of pathology and pathogenesis of the main disease of human: Commencement address. - Tashkent, 2007. - P. 6-8.
- Agapov M.Y., Khamoshin A.V. Screening of colorectal cancer: Methodic elaboration for physicians. - Vladivostok, 2002. - 28 p.
- Axel E.M., Davydov M.I., Ushakova T.I. Malignant neoplasms of gastro-intestinal tract: main statistical indicators and trends // Modern oncology. - 2001. - № 4. - P. 141-145.
- An V.K., Rivkin V.L. Urgent proctology. - Medicine, 2002.
- Analysis of somatic K-ras mutations in colonic polyps // Sazonova M.A., Vaganov Y.E., Korchagina E.L. et. al. // Medical Genetics. - 2005. - № 6. - P. 263.
- Anichkov N.M., Kvetnoy I.M., Konovalov S.S. Biology of neoplastic growth (molecular-medical aspects). - Sanct-Petersburg, Prime Euro-Sign, 2004.
- Aruin L.I., Kapuller L.L., Isakov V.A. Morphological diagnostics of diseases of stomach and intestine. - Мedicine, 1998. - P. 412-450.
- Babin V.A., Mushkin O.N., Dubinin A.V. Molecular aspects of symbiosis in host-micro flora system // Russian Journ. Hepatol. Coloproctol. - 1998. - № 6. - P. 76-82.
- Barsoukov Y.A., Knysh V.I. Modern opportunities of treatment of colorectal cancer // Modern oncol. - 2006. - Vol. 8, № 2. - P. 7-11.
- Garkavtseva R.F., Kozoubskaya T.P. Genetics of gastro-intestinal tract cancer // Clinical Oncology. Мedicine. - 2002. - № 2. - P. 12-15
- Kniazev M.V. Is it possible to reduce colorectal cancer morbidity // Attending doctor. - 2003. - № 2. - P. 31-34.
- Pobedinskiy A.A. The role of colonoscopy in diagnostics and treatment of colonic polyps // Adaptation-compensation mechanisms of regulation of body functions in the modern environment conditions: International conference. - Gomel, 2000.
- Portnoy L.M. The place of modern traditional radiology in diagnostics of colonic tumors // Methodical textbook. - 2000. - Vol. 27. - P. 11.
- The spectrum of somatic mutations in APC genes, k-Ras and TP53 in Russian patients with colorectal cancer and precancerous diseases of colon / Kostin P.A., Generosov E.V., Zakharzhevskaya et al. // Russian Journ. Gastroenterol. Hepatol., Coloproctol. - 2008. - № 4. - P. 53-62.
- Yakoutin N.A., Gorban V.A., Zozoulia M.V. Diagnostics of precancerous diseases and initial forms of colic cancer at pre-admission stage // Problems of Coloproctology. - Мedicine, 2002. - P. 502-507.
- Adler G., Fiocchi C., Vorobiev G.J., Lasebnik L.B. Inflammatory Bowel Disease-Diagnostic and Therapevtic Stratigies // Falk Symposium 154. - 2007. - P. 237.
- Akemi Ito. Indications and limitations of endoscopic surgery on colorectal tumors Digestive Endoscopy. - 2000. - Vol. 12. - P. 16.
- Belous Т.А. Pathomorphology of precancerous conditions of colon // Russian J. Of Gastroenterology, Hepathology and Coloproctology. - 2002. - № 4.0. - P. 50-56.
- Bond J.H. Polyp Guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps // Amer. J. Gastroenterol. - 2000. - Vol. 95, № 11. - P. 46-54.
- Bories E., Pesenti C., Monges G., Lelong B., Moutardier V., Delpero J.R., Giovannini M. Endoscopic mucosal resection for advanced sessile adenoma and early-stage colorectal carcinoma // Endoscopy. - 2006. - № 38. - P. 231-235.
- Cherkasov M.F. Opportunities of screening method in colorectal cancer case finding// Actual issues of Coloproctology. M.: Medicine, 2006.
- CT colonography predictably overestimates colonic length and distance to polyps compared with optical colonoscopy / Duncan J.E., McNally M.P., Sweeney W.B. et al // AJR Am J. Roentgenol. - 2009. - Vol. 193, № 5. - P. 1291-5.
- CT colonography: accuracy of initial interpretation by radiographers in routine clinical practice / Burling D., Wylie P., Gupta A. et al // Clin Radiol. - 2010. - Vol. 65, № 2. - P. 126-32.
- Endoscopic mucosal resection for colonic non-polypoid neoplasms / Ning-Yao Su, Chen-Ming Hsu, Yu-Pin Ho et al. // Amer J. Gastroenterol. - 2005. - Vol. 100. - P. 2174-2179.
- Greenhalh T. Basics of Evidence Based Medicine / Transl. from Engl. - M.: Geotar-Med, 2004. - 240 p.
- Identification of a chromosome 18q gene which is altered in colorectal cancer / Fearon E.R., Cho K.R., Nigro J.M. et al. // Science. - 1990. - Vol. 247. - P. 49-56.
- Khomoutova E.Y., Ignatiev Y.T. Multispiral computed virtual colonoscopy in diagnostics of colonic pathology (Review) // Med. Visulisation. - 2008. - № 5. - P. 73.
- Ming-Yao Su, Chen-Ming Hsu, Yu-Pin Ho et al. Endoscopic mucosal resection for colonie non-polypoid neoplasma // Ann. J. Gastroenterol. - 2005. - Vol. 100. - P. 2174-2179.
- Endoscopic mucosal resection for colonic non-polypoid neoplasms / Ning-Yao Su, Chen-Ming Hsu, Yu-Pin Ho et al. // Amer J. Gastroenterol. - 2005. - Vol. 100. - P. 2174-2179.
- Nakajima T. Problem of total colonoscopy for mass screening of colorectal cancer // Dis. Colon. Rectum. - 2004. - Vol. 47. - P. 1052.
- Pickhard P.J. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults // New Engl. J. Med. - 2003. - Vol. 349. - P. 2191-2200.
- Portielje J.E.A. IL-12: a promising adjuvant for cancer vaccination // Cancer Immunol. Immunother. - 2003. - Vol. 52. - P. 133-144.
- Rivera M. Virtual colonoscopy // Gastroenterology. - 2003. - Vol. 3. - P. 284-287.
- Rubito C.A. Classification of Colorectal Polyps: Guidelines for the Endoscopist // Endoscopy. - 2002. - Vol. 112. - P. 226-236.
- Suuzuk K., Rockey D.C., Dachman A.H. CT colonography: advanced computer-aided detection scheme utilizing MTANNs for detection of "missed" polyps in a multicenter clinical trial // Med Phys. - 2010. - Vol. 37, № 1. - P. 12-21.
- Thornton E., Morrin M.M., Yee J. Current status of MR colonography // Radiographics. - 2010. - Vol. 30, № 1. - P. 201-18.
- Virtual colonoscopy: procedure / Khomoutova E.U., Ignatieva Y.T., Skripkin D.A., Phillipova Y.G. // Radiology - Practice. - 2009. - № 2. - P. 21-27.

