The synthesis and study of the physicochemical properties of complex compounds of rare earth elements with heterocyclic compounds is a promising direction in the chemistry of coordination compounds [1, 2].
Earlier, we [3] performed work on the synthesis of complex compounds of chlorides of a number of lanthanides (Ln = La3 +, Nd3 +, Pr3 +, Sm3 +, Gd3 +, Dy3 +, Er3 +) with nicotinamide.
Experimental part
A physicochemical study of the interaction of lanthanum nitrate with nicotinamide was carried out in an aqueous medium by the isothermal solubility method at 25 °C. The concentration of lanthanum ions and the presence of nicotinamide nitrogen were determined by traditional methods [4, 5].
La(NO3)3-C6H6N2O-H2O system at 25 °C
According to the results of the experimental data of the study of the system lanthanum nitrate – nicotinamide – water, a solubility diagram was constructed (table 1, fig. 1), consisting of three crystallization branches.
Table 1
Experimental data on solubility in the system La(NO3)3-C6H6N2O-H2O at 25 °C
|
№ |
Liquid phase, in mass % |
Solid phase, in mass % |
Crystallizing phase |
||
|
La(NО3)3 |
C6H6N2O |
La(NО3)3 |
C6H6N2O |
||
|
1 |
58,78 |
- |
75,05 |
- |
La(NО3)3•6H2O |
|
2 |
56,41 |
5,21 |
69,22 |
1,81 |
|
|
3 |
56,17 |
8,83 |
70,01 |
2,32 |
|
|
4 |
57,08 |
14,22 |
68,81 |
5,08 |
|
|
5 |
57,10 |
14,23 |
77,75 |
15,85 |
La(NО3)3•6H2O + La(NO3)3•2C6H6N2O•2H2О |
|
6 |
57,10 |
14,23 |
54,45 |
34,45 |
La(NO3)3•2C6H6N2O•2H2О |
|
7 |
50,63 |
13,01 |
52,88 |
31,90 |
|
|
8 |
45,11 |
12,17 |
50,95 |
30,93 |
|
|
9 |
29,90 |
13,85 |
45,32 |
30,85 |
|
|
10 |
24,20 |
17,49 |
44,38 |
33,18 |
|
|
11 |
21,85 |
19,73 |
45,46 |
34,92 |
|
|
12 |
19,31 |
23,22 |
46,72 |
36,75 |
|
|
13 |
18,10 |
25,11 |
42,40 |
35,45 |
|
|
14 |
17,02 |
27,65 |
40,90 |
35,91 |
|
|
15 |
16,02 |
30,61 |
42,38 |
37,08 |
|
|
16 |
14,10 |
44,98 |
44,93 |
39,90 |
|
|
17 |
14,09 |
45,01 |
47,93 |
40,95 |
|
|
18 |
14,07 |
45,01 |
42,01 |
53,01 |
La(NO3)3•2C6H6N2O•2H2О + C6H6N2O |
|
19 |
14,09 |
45,01 |
24,01 |
66,96 |
|
|
20 |
14,08 |
45,35 |
4,24 |
82,91 |
C6H6N2O |
|
21 |
11,89 |
44,43 |
3,98 |
81,04 |
|
|
22 |
7,93 |
43,81 |
2,21 |
83,95 |
|
|
23 |
2,89 |
44,38 |
1,02 |
82,05 |
|
|
24 |
- |
45,65 |
- |
- |
|
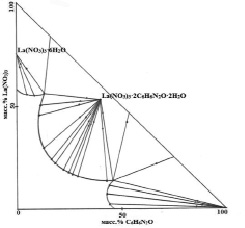
Fig. 1. Solubility isotherm of the La(NO3)3-C6H6N2O-H2O system at 25 °C
The first branch of crystallization corresponds to the separation of lanthanum hexahydrate nitrate into the solid phase. The solubility of lanthanum nitrate at 25 °C is 58.78 %.
Upon reaching the concentration of lanthanum nitrate – 57.10 %, nicotinic acid amide – 14.23 %, water – 28.67 %, a new compound congruently soluble in water (the second crystallization branch) of the composition 1: 2: 2 (La(NO3)3:C6H6N2O:H2O). The next branch (the third branch) corresponds to the crystallization of pure nicotinamide.
By the nature of the diagram of the ternary system La(NO3)3-C6H6N2O-H2O at 25 °C, one can judge that complexation occurs between the reacting components, that is, the formation of one compound corresponding to the gross formula La (NO3) 3 • 2C6H6N2O • 2H2O.
To determine the pycnometric density of crystals of the obtained compound, the solubility of the complex in organic solvents has been studied.
From the obtained experimental data, it can be seen that the compound under study is soluble in alcohol, acetone, ether and practically insoluble in benzene. Using an indifferent liquid (benzene), the density of solid phases was determined by the pycnometric method (d = 1,46 g⁄см3).
In order to identify a new compound, to clarify the nature of the chemical bond in the complex, IR absorption spectra of nicotinamide and its coordination compound with lanthanum nitrate in the range of 400-4000 cm-1 were investigated on a Nicolet-IR-1200 spectrometer in the form of tablets with potassium bromide (fig. 2, 3). The values of the characteristic frequencies in the IR absorption spectrum are given in table 2.
Table 2
Solubility of nicotinamide and a complex compound in organic solvents, wt %
|
Connections |
In alcohol |
in acetone |
on air |
in benzene |
d (g/см3) |
|
C6H6N2O |
21,43 |
14,61 |
1,62 |
н.р |
1,38 ± 0,04 |
|
La(NO3)3•2C6H6N2O•2H2О |
14,77 |
8,12 |
1,96 |
н.р. |
1,46 ± 0,03 |
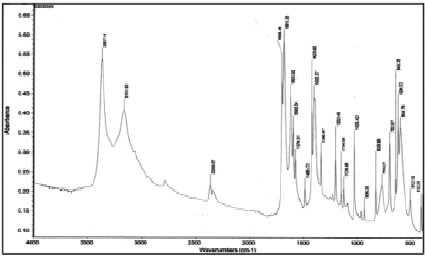
Fig. 2. IR absorption spectrum of nicotinamide
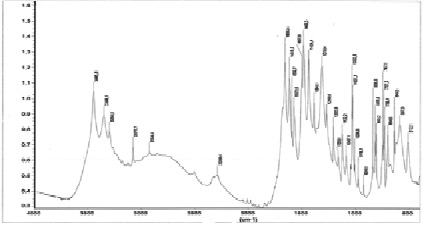
Fig. 3. IR absorption spectrum of the complex La(NO3)3•2C6H6N2O•2H2О
In the IR absorption spectrum of the nicotinamide complex of lanthanum nitrate, a shift of the stretching vibration ν (C = O) to the low-frequency region from 1682 cm-1 to 1656 cm-1 is observed, and the stretching vibrations of the displacement ring do not experience. The absorption bands related to stretching vibrations n (C-N) are shifted towards higher frequencies from 1340 cm-1 to 1384 cm-1, which indicates an increase in the multiplicity of the C-N bond and a weakening of the C = O bond.
The bands of bending vibrations of the δ (NH2) -group are slightly shifted towards low frequencies from 1619 cm-1 to 1615 cm-1, which is explained by the strengthening of the C-N bond. In the 1600-1700 cm-1 region, the δ (H2O) bands appear, overlapping with the intense n (CO) and n (NH) bands.
Such changes in the positions of the bands “amide 1” and amide 2 “suggest the coordination of nicotinic acid amide to lanthanum ions through the oxygen atoms of the carbonyl group.
In the region of stretching vibrations ν (N-H), a broad spectrum is observed in the spectrum of the complex.
Band with indistinct maxima at 3349, 3077 cm-1, which is associated with overlapping valence bands ν (OH) and ν (N-H), indicating the hydration of the compound.
Table 3
Experimentally obtained frequencies of stretching and bending vibrations of nicotinic acid amide and the newly obtained compound
|
Assignment |
С6Н6N2O |
La(NО3)3•2C6H6N2О•2H2О |
|
νas (NH2), ν (ОН-) |
3367 |
3349 |
|
νs (NH2) |
3164 |
3077 |
|
v(С=О) |
1682 |
1656 |
|
δ(NH2), δ(H2О) |
1619 |
1615 |
|
ν (pyridine ring) |
1593 1574 |
1593 1572 |
|
ν(pyridine ring), δ (ССН) |
1485 |
1482 |
|
v (СN) |
1340 |
1384 |
|
δ (ССН) |
1202 |
1204 |
|
v (pyridine ring) |
1029 |
1027 |
|
ν(CC), δ (ССC) |
829 |
838 |
|
δ (ССN), δ(СO) |
703 |
699 |
Based on the data obtained, the complex compound of lanthanum with nicotinic acid amide can be assigned the following structure:
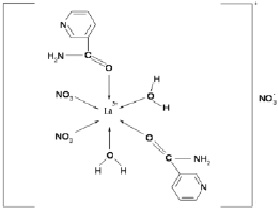
Fig. 4. Scheme of the structure of the complex compound La(NO3)3∙2С6Н6N2O∙2H2O
Thus, the analysis of the IR spectra of nicotinamide and the new complex compound showed that the nicotinamide molecules in this complex act as a monodentate ligand, coordinating through the oxygen atoms of the carbonyl group of nicotinic acid amide.
In order to obtain information about the crystal structure of the obtained complex, an X-ray study of the compound of lanthanum with nicotinic acid amide was carried out on a DRON-3.0 diffractometer (CoKα radiation, with a filter). The results are shown in Fig. 5, 6, tabl. 4, 5.
Table 4
X-ray analysis of nicotinamide С6Н6N2O
|
2θ |
H |
Ө |
I % |
d(A0) |
H |
k |
L |
Syngonia |
|
16,80 |
132 |
8,40 |
94 |
6,1274 |
0 |
0 |
1 |
Monoclinic а = 7,051 b = 11,338 c= 6,551 β = 1100 |
|
22,72 |
68 |
11,36 |
48,5 |
4,5443 |
0 |
2 |
1 |
|
|
25,53 |
140 |
12,76 |
100 |
4,0527 |
0 |
2 |
1 |
|
|
27,40 |
17 |
13,70 |
12 |
3,7794 |
0 |
3 |
0 |
|
|
28,70 |
33 |
14,35 |
23 |
3,6116 |
1 |
1 |
1 |
|
|
29,80 |
101 |
14,90 |
72 |
3,4811 |
1 |
1 |
1 |
|
|
31,50 |
89 |
15,75 |
63 |
3,2976 |
2 |
0 |
0 |
|
|
34,90 |
31 |
17,45 |
22 |
2,9849 |
0 |
4 |
0 |
|
|
37,75 |
12 |
18,87 |
8 |
2,7676 |
0 |
2 |
2 |
|
|
39 |
23 |
19,50 |
16 |
2,6815 |
0 |
4 |
1 |
|
|
39,85 |
27 |
19,92 |
19 |
2,6272 |
0 |
4 |
1 |
|
|
41,55 |
22 |
20,77 |
16 |
2,5341 |
2 |
1 |
1 |
|
|
42,93 |
62 |
21,46 |
44 |
2,4466 |
2 |
1 |
1 |
|
|
44,95 |
36 |
22,47 |
26 |
2,3420 |
1 |
1 |
2 |
|
|
47,70 |
26,5 |
23,85 |
19 |
2,21372 |
1 |
2 |
2 |
|
|
55,65 |
17 |
27,82 |
12 |
2,01796 |
0 |
0 |
3 |
|
|
59,28 |
19 |
29,64 |
13 |
1,80994 |
3 |
0 |
1 |
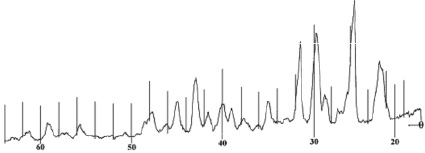
Fig 5. X-ray of nicotinamide С6Н6N2O
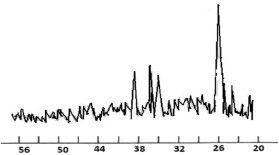
Fig. 6. X-ray diffraction pattern of a complex compound La(NO3)3·2С6Н6N2O·2Н2O
Table 5
X-ray analysis La(NO3)3∙2С6Н6N2O∙2H2O
|
№ |
2Ө |
H |
Ө |
I( %) |
D(Å) |
h |
k |
l |
Syngonia |
|
1 |
9,1 |
41 |
4,55 |
100 |
11,2791 |
0 |
0 |
1 |
Rhombic a = 8,805 b = 8,980 с = 11,279 |
|
2 |
11,43 |
34 |
5,72 |
82 |
8,9859 |
0 |
1 |
0 |
|
|
3 |
11,66 |
20 |
5,83 |
48 |
8,8091 |
1 |
0 |
0 |
|
|
4 |
14,66 |
30 |
7,33 |
73 |
7,0141 |
1 |
1 |
1 |
|
|
5 |
15,13 |
36 |
7,57 |
87,8 |
6,7983 |
0 |
0 |
2 |
|
|
6 |
16,80 |
14 |
8,40 |
34,14 |
6,1259 |
0 |
2 |
0 |
|
|
7 |
16,96 |
14 |
8,48 |
34,14 |
6,0698 |
2 |
0 |
0 |
|
|
8 |
17,63 |
9 |
8,82 |
21,95 |
5,8401 |
2 |
2 |
2 |
|
|
9 |
18,16 |
8 |
9,08 |
19,50 |
5,6717 |
2 |
3 |
1 |
|
|
10 |
19,91 |
10 |
9,96 |
24,39 |
5,1779 |
0 |
0 |
3 |
|
|
11 |
21,61 |
11 |
10,81 |
26,82 |
4,7746 |
3 |
0 |
3 |
|
|
12 |
22,10 |
13 |
11,05 |
31,70 |
4,6699 |
3 |
0 |
0 |
|
|
13 |
22,41 |
8 |
11,21 |
19,50 |
4,6063 |
3 |
3 |
3 |
|
|
14 |
23,55 |
7 |
11,78 |
17,07 |
4,3862 |
3 |
1 |
2 |
|
|
15 |
23,83 |
12 |
11,92 |
29,26 |
4,3352 |
0 |
0 |
4 |
|
|
16 |
24,16 |
10 |
12,08 |
24,39 |
4,2762 |
0 |
4 |
0 |
|
|
17 |
25,83 |
8 |
12,92 |
19,50 |
4,0045 |
4 |
0 |
0 |
|
|
18 |
26,66 |
11 |
13,33 |
26,82 |
3,8820 |
4 |
4 |
4 |
|
|
19 |
27,16 |
10 |
13,58 |
24,39 |
3,8118 |
4 |
0 |
4 |
|
|
20 |
27,100 |
23 |
13,55 |
56,09 |
3,8199 |
4 |
1 |
2 |
|
|
21 |
29,16 |
15 |
14,58 |
36,58 |
3,5551 |
4 |
3 |
2 |
|
|
22 |
30,100 |
12 |
15,05 |
29,26 |
3,4469 |
0 |
0 |
5 |
|
|
23 |
31,16 |
12 |
15,58 |
29.26 |
3,3321 |
5 |
0 |
5 |
|
|
24 |
31,80 |
9 |
15,90 |
21,95 |
3,2670 |
5 |
0 |
0 |
|
|
25 |
33,25 |
4 |
16,63 |
9,75 |
3,1283 |
0 |
5 |
0 |
|
|
26 |
33,58 |
5 |
16,79 |
12,19 |
3,0985 |
5 |
5 |
5 |
|
|
27 |
33,100 |
10 |
16,55 |
24,39 |
3,1420 |
1 |
2 |
3 |
|
|
28 |
35,83 |
5 |
17,92 |
12,19 |
2,9096 |
4 |
4 |
4 |
|
|
29 |
36,33 |
7 |
18,17 |
17,07 |
2,8709 |
5 |
3 |
4 |
|
|
30 |
37,50 |
6 |
18,75 |
14,63 |
2,7843 |
0 |
0 |
6 |
|
|
31 |
37,83 |
5 |
18,92 |
12,19 |
2,7610 |
0 |
6 |
0 |
|
|
32 |
38,50 |
6 |
19,25 |
14,63 |
2,7146 |
6 |
0 |
0 |
|
|
33 |
39,30 |
6 |
19,65 |
14,63 |
2,6617 |
6 |
1 |
6 |
|
|
34 |
39,60 |
5 |
19,80 |
12,19 |
2,6421 |
6 |
2 |
3 |
|
|
35 |
41,16 |
6 |
20,58 |
14,63 |
2,5462 |
6 |
0 |
6 |
The X-ray diffraction pattern of the complex contains new lines characteristic of the compound, and the lines of the initial components are absent.
By indicating the main parameters of the diffractogram, we obtained the following unit cell parameters: a = 8.805; b = 8.980; c = 11.279 and we assume that the crystal lattice of the test sample is assigned to the rhombic system.
Библиографическая ссылка
Osmonova S.S., Shamatova B.O. PHYSICAL AND CHEMICAL ANALYSIS OF THE SYSTEM OF LANTHANUM NITRATE – NICOTINAMIDE – WATER 25 °С // European Journal of Natural History. 2021. № 6. С. 3-8;URL: https://world-science.ru/ru/article/view?id=34204 (дата обращения: 05.01.2026).

