At the present stage, the development of the human eye has been studied to a greater extent on animal models. The problems of congenital pathologies of the human eye are one of the main aspects studied in modern most relevant research. One of the little-studied issues of eye development is the study of the mechanisms of retinal development, the participation of macrophages in the formation of the vascular system of the eye, migration of neurons and glia in the formation of retinal layers. The upper and lower eyelids merge during development and then separate again. The mechanism for re-detaching the eyelids is still unclear. These questions determined the direction of our research, since they can contribute to solving the problem of angiogenesis and vascular involution.
Choroidal neovascularization (CNV) directly related to the loss of vision in certain eye diseases such as age-related macular degeneration. Although several histological studies in humans have confirmed the involvement of macrophages in the formation of CNV, the exact mechanisms are still not fully understood, the submission can only be based on a consideration of this process in human ontogenesis. Comprehensive insights can enable the control of key targets in these complex eye development processes [1].
Even in the wall of the yolk sac, the mechanism for the formation of blood vessels and blood cells is triggered. Embryonic hematopoiesis is a process of development and renewal of blood; it begins in the first hours after fertilization and continues in the early stages of human prenatal development. During the entire period of antenatal development, there is a massive death and renewal of blood cells. In this case, dead cells are replaced with new ones in an equivalent ratio. During hematopoiesis, leukocytes are produced in the first hours of life, but we will focus on the reproduction of monocytes, which in the process of their development become tissue macrophages. In postembryonic development, the activation of monocytes is enhanced under the action of a variety of signaling molecules that cause the differentiation of monocytes into various functional types. After the formation of monocytes, no more than 5% of their population enters the blood, where it circulates from 8 hours to 4 days, the rest is in the extravascular pool in order to turn into various types of macrophages and acquire new specialization, for example, in the nervous tissue, monocytes turn into microglia. All this is a single monocytic-macrophage system.
Microglia cells originate directly from blood monocytes or perivascular macrophages, that is, they are characterized by mesodermal origin. Microglia are cells of non-neuronal origin that are found in the central nervous system. Thus, they can also be attributed to the macrophage-monocytic system. Microglial cells have a high ability to migrate and multiply. Inactivated microglia are small cells with characteristic processes with perpendicularly branching branches, which make it possible to recognize various agents in their environment just with the help of specialized membrane receptors located on the processes. During apoptotic and inflammatory processes, microglia are activated, while the shape of the cells undergoes strong changes. When activated, they release numerous processes that resemble amoeba lamellipodia [2].
Researchers Tao Huang, Jianlin Cui, Lei Li, Peter F Hitchcock and Yuhao Li indicate that in zebrafish, early macrophages migrate from the yolk sac to the brain and retina 26-30 hours after fertilization (hpf) and transform into microglia after 55-60 hpf. The migration of macrophages into the central nervous system requires signaling by the receptor for factor-1 stimulating macrophage colonies (csf-1r), which is encoded by the fms gene. It has been established that targeted knockdown of csf-1r morpholino oligonucleotides delays the migration of macrophages from the yolk sac to the retina, and this delay in macrophage migration leads to microphthalmia, delayed exit from the cell cycle among retinal progenitors, and lack of neuronal differentiation. If embryos survived under conditions of morpho-dependent inhibition, translation was lost, and microglia re-migrated into the retina, and neuronal differentiation was partially restored. However, there is no reliable data on whether microglial migrants had phagocytic activity? To solve this problem, further deeper research is required [3]. It is not excluded that vitamins of the B group may be of great importance for phagocytic activity, namely folic acid, which is necessary for the synthesis of nucleoproteins and promotes the processes of maturation and cell division. With a deficiency of B vitamins and internal Castle factor, dystrophy of human retinal cells may develop in prenatal ontogenesis. Maintaining the process of synthesis and timely maturation of monocytes is a complex, genetically determined system. At the same time, serious violations of this process lead to abnormal development of the vital structures of the human eye [4].
Purpose of our research was to obtain new data and information on the role of macrophages in the morphogenesis of the human retina, contributing to the development of pathogenetically based strategies in the prevention and treatment of disorders of the development of the organ of vision.
Material and methods
The study was performed taking into account the provisions of the Helsinki Declaration (2000, 2013) and with the permission of the ethical committee of the Far Eastern Federal University. The localization of CD163 positive cells in the dynamics of development of the structures of the human eye was revealed using the material of 15 human embryonic and fetal eyes by the method of immune histochemistry. Stained sections of biopsy specimens were performed according to classical protocol for a method using hematoxylin and eosin.
Results of analysis of literature data
It is known that the only ocular field is formed in the center of the front of the neural plate during gastrulation; it is characterized at the molecular level the expression “eye field transcription factors”. The single field of the eye is divided into two, forming the optic vesicle, and then (under the influence of the lens placode) the optic cup. The lens develops from the lens placode (superficial ectoderm) under the influence of the underlying optic bubble. PAX6 acts as a major control gene in this phase and activates genes encoding cytoskeletal proteins, structural proteins, or membrane proteins. It is known that the endothelial growth factor is affiliated with PAX1, which is responsible for the axial organization of the human embryo organism; therefore, it is functionally linked directly to macrophages that secrete VEGF [5]. The cornea forms from the superficial ectoderm, and cells from the periocular mesenchyme migrate into the cornea, giving rise to the future corneal stroma. In the same way, the iris and ciliary body are formed from the optic cup. The outer layer of the optic cup becomes pigmented retinal epithelium, while the bulk of the inner layer of the optic cup later forms the neural retina with six different cell types, including photoreceptors. Although fish, unlike higher vertebrates, are capable of growth throughout their life, the environment is also an important factor in controlling the development of eyes in this group, and eye development is not strictly genetically determined [6].
Many structures in the developing eye are temporary and undergo involution during development, including through apoptosis. Cells undergoing apoptosis look like oval clusters with intensely stained cytoplasm and dense chromatin contained in the nucleus. The shrinking of the cell, and then the lysis of the organelles leads to the formation of apoptotic particles, which are rapidly phagocytosed, after which they disintegrate and are thrown into the lumen of the organ. On histological preparations, apoptosis can be detected only in cases of its severity [7]. Unlike necrosis, apoptosis is not accompanied by an inflammatory reaction, which also complicates its histological identification. The apoptosis of cells of these structures and their phagocytosis are triggered with the participation of macrophages (fig. 1).
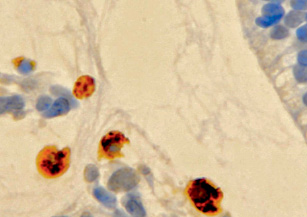
Fig. 1. Macrophages in the developing vitreous humor of a human fetus for 10 weeks. Immune histochemistry for the detection of CD68 positive cells. Phagocytosis of children of the mesenchymal vitreous body. Magnification x400
Tunica vasculosa lentis (TVL) is a temporary vasculature surrounding the developing lens that regresses prenatally in humans and is a prime example of regulated involution. Research has shown that macrophages play an active role in initiating and completing the process of programmed cell death during human eye development. Macrophages are called professional phagocytes because their main role is phagocytosis. The process of phagocytosis in the structures of the human eye is complex and poorly understood today. It has also been suggested that macrophages around the developing lens are likely to migrate into the neural retina and differentiate into microglia after completing their role as removal [8].
The retinal ganglion cells grow in the direction of the optic nerve to form the optic tract. Cellular processes during eye development known in frogs, zebrafish, chickens, and mice, as well as established differences between species, represent directions in the study of missing links for future research. It was found that macrophages expressing CD163, located in the ectomesenchyme surrounding the optic vesicle, were detected in the head section of the human embryo from the end of the 3rd week. The presence of macrophages with expression of CD163 is due to the fact that the development of human eye structures depends on the main induction mechanism of differentiation, which is realized through the interaction of macrophages and cells of the forming eye vesicle. Macrophages secrete TGF-beta, are able to phagocytose IgG-associated latent TGF-beta complexes, and release active TGF-beta into the extracellular matrix to induce apoptosis of various cell types through signaling pathways: SMAD and DAXX. TGF-beta activation depends on various factors that activate signaling pathways. We noted that in areas with high proliferative activity, cells with the CD163 phenotype are absent, earlier being detected in the inner layers of the retina, which indirectly confirms their main role at this stage in the supply of signaling molecules specifically for differentiating cells, for the appearance of various repression of cell genomes and directed induction of cell differentiation in various tissues of the human eye [9].
Complex immunological relationships based on the principles of direct and feedback arise and form between the body of the mother and the fetus. These relationships ensure correct harmonious development. The relevance of the study of the development of the human eye is the basis of immunological studies and can reveal most of the causes that are complications in the development of the human eye. For this purpose, clinical studies of quantitative indicators of two parameters of cellular health are being tested: the activity of the production of intracellular nitric oxide (by the fluorescence of the oxidative dye DAX-J2 Orange) and cell death (by the penetration of 7-AAD cells). With the introduction of this method into work, it is possible to obtain statistically reliable results in terms of the percentage and concentration of cells: living, living with active nitric oxide, dead, dead with active nitric oxide for the total number of cells with high nitric oxide activity, which can be one of the functional criteria. the activity of the immune system of the mother and the developing fetus [10].
Both apoptotic cells and macrophages are identified in the area of the eyelid junction and during cell proliferation (fig. 2). In the tissues of the choroid, in the formation zone of the anterior chamber of the eye, TUNEL-positive cells and immunohistochemically positive macrophages were also found at this stage. This suggests that apoptosis, like phagocytosis, can also play an important role in providing space for the proliferation of newly organized epidermal cells without wide intercellular spaces. In addition, it is recognized that the progressive differentiation of epithelial cells from the skin of the eyelids to the connective region of the conjunctiva may also play a role in eyelid separation [11].
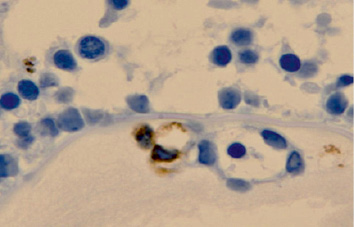
Fig. 2. Apoptosis of the eye structures of a human fetus at 14 weeks. Vascular involution. Immune histochemistry to identify cells expressing CD68. Magnification x400
The data of our study expand the diagnostic resource for identifying the causes of congenital retinal pathology in premature infants and are necessary to create a fundamental platform in the development of new, more effective and pathogenetically determined conservative methods of treatment and prevention of eye pathology in newborns [12].
Conclusion
The macrophage-monocytic system is essential for normal growth and development of the human retina and neurogenesis. It is known that normally microgliocytes are evenly distributed in the cortex and, moreover, in a checkerboard pattern, like protoplasmic astrocytes. The area of distribution of processes of one microgliocyte does not go over to the territory of an adjacent gliocyte. Recently, it has been shown that each microgliocyte with its processes is associated with certain nerve and glial cells and vessels. The main function of microgliocytes is phagocytosis. The “eaten” mass usually consists of cellular waste products, lipids and apoptotic bodies in a non-inflamed state, as well as inflamed areas damaged by viruses, bacteria or other agents. As soon as the microglial cell is “filled”, it goes into an inactive state for processing the material. This study provides new insights into the neurogenic role of macrophages.
Macrophages secreting endothelial growth factor (VEGF) regulate angiogenesis in the structures of the human eye during ontogenesis.
Inflammation of the organ of vision is associated with an increased influx of phagocytic cells. According to our data, it can be assumed that subpopulations of macrophages perform different functions in the induction of apoptosis and phagocytic activity in physiological regeneration and under conditions of pathological development of the organ of vision.
The study was financially supported by the International Medical Research and Education Center (Vladivostok, Russia).
Scientific advisers – Doctor of Medical Sciences, Professor G.V. Reva; Doctor of Technical Sciences, Professor A.N. Gulkov.
Библиографическая ссылка
Можилевская Е.С., Садовая Я.В., Пономарев А.В., Ленда И.В., Бессонов Е.А., Салатов Я.С. РОЛЬ МАКРОФАГОВ В РАЗВИТИИ СТРУКТУР ГЛАЗА ЧЕЛОВЕКА В ПРЕНАТАЛЬНОМ ОНТОГЕНЕЗЕ // European Journal of Natural History. 2021. № 4. С. 27-31;URL: https://world-science.ru/ru/article/view?id=34194 (дата обращения: 27.02.2026).

