The binary and the complex chalcogenides of copper are among the important functional materials of modern technology. Many of these class compounds are semiconductors, photo-, seqneto- and thermoelectric materials, solid superion conductors etc. as widely used or considered perspectively for the application [1–11].
It has been understood from researches in the Cu–Sn–S system in the past 40 years that more than 20 compounds are obtain in this system and interesting compounds are observed in some of these compounds. The thin layers of compounds containing Cu3SnS4, Cu2SnS3 and Cu4SnS4 have p-type conductivity and it was considered new and perspective materials for the solar energy converter devices. It allows the establishment of new generation thermoelectric devices based on them detection of good thermoelectric properties in compounds containing Cu2SnS3 and Cu4Sn7S16 [7, 9, 11].
The compounds Cu3SnS4, Cu2SnS3, Cu4SnS4, Cu2Sn3S7, Cu2Sn3,5S8, Cu2Sn3,75S8, Cu2Sn4S9, Cu2Sn3,34 S7,68 and Cu4Sn7S13 present in the Cu–Sn–S system are stable at room temperature. Cu10Sn12S13 400 °C is above, Cu5Sn2S7 and Cu7Sn3S10 are stable above 600 °C. The pseudobinar Cu2S-SnS2 system has been found to have four compounds: Cu2SnS3, Cu4SnS4, Cu2Sn3S7 and Cu4Sn3S8. Cu2SnS3, Cu4SnS4 and Cu2Sn3S7 from these compounds are stable at room temperature. The Cu4Sn3S8 compound can be available at a temperature range of 685–785 °C. Some physical-chemical properties of these compounds – crystal structures, melting and polymorphic conversion temperatures were investigated via X-ray and DTA methods [1–11].
The element compounds or binary compounds (Cu2S v∂ SnS2) of thiostannates of copper are synthesized by melting (1200 °C) together in vacuumed (~10-2 Pa) quartz ampoules. In some studies [10] Cu2SnS3 and Cu3SnS4 containing compounds were obtained hydro- and solvothermal method and their optical properties were investigated. Deposition conditions and endurance limits of intermediate phases were investigated in Cu–Sn–O–H system in aqueous solution [8].
Nowadays, obtaining the thiostannates by chemical precipitation from aqueous solution and learning their properties is one of the most actual issues. Generally, nano-sized particles of the substances are formed in the thin layers obtained by chemical precipitation from aqueous solution. As is known, many physical-chemical properties of nano-particles are differ from properties of dense materials.
The aim is to investigate the obtaining conditions for the Cu2SnS3 Cu4SnS4 and Cu2Sn3S7 compounds using the aqueous solutions of CuCl, SnCl4 and CH3–CS–NH2 compounds.
In the article the results of the synthesis of Cu2SnS3 Cu4SnS4 and Cu2Sn3S7 compounds from aqueous solution by hydrochemical method were given by X-ray, DTA and scanning electron microscopic analysis methods.
Experimental part and discussion of the results
Three samples were taken from the 0.1 M SnCl4 solution for the obtain Cu2SnS3 Cu4SnS4 and Cu2Sn3S7 compounds. CuCl have been resolved in the SnCl4 solutions by weighing appropriate amount of to the stoichiometric structures of the proper compounds in the analytical scale. Then 0.1 M CH3–CS–NH2 solution was added in stoichiometric quantities on each samples in the inert condition (N2), the pH of the condition was raised to 6 with 0.1 M NH4OH solution and the solution was stirred for 30 minutes at 70 °C with a magnetic stirrer. The molar ratio of the elements in the solutions has been like Cu:Sn:S=2: 1:3; 4:1:4 and 2:3:7. The following device was used to make the experience (in N2 atmosphere) (fig. 1).
Acquired sediments was filtered, firstly washed with pure water, then with ethanol, then dried in the vacuum at (~10-1 Pa) 80 °C for a hour. The dried sediments were thermally processed for 2 hours in quartz ampoules (~10-2 Pa) vacuumed at 400 °C. Reaction equations can be written as follows when occur during the acquire of Cu2SnS3 Cu4SnS4 and Cu2Sn3S7 compounds:
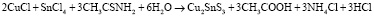 ,
,
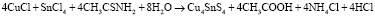 ,
,
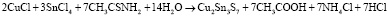 .
.
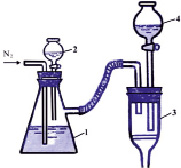
Fig. 1. Scheme of the device used to acquire Cu2SnS3 Cu4SnS4 and Cu2Sn3S7 compounds [6]: 1 – mixture of SnCl4 and CuCl which it is in the flask; 2 – the funnel drop filled with solution of CH3–CS–NH2; 3 – funnel to filter the sediment; 4 – funnel to clean down
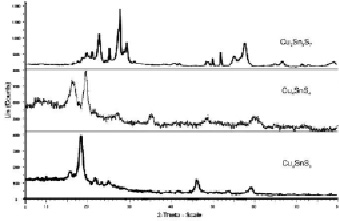
Fig. 2. Diffractograms of Cu2SnS3 Cu4SnS4 and Cu2Sn3S7 compounds
The individuality of the obtined compounds has been confirmed through RFA (2D PHASER “Bruker”, CuKa, 2θ, 20–80 deg.) and DTA (pyrometr НТР – 70, device Термоскан-2, inert condition) methods (Fig. 2). According to X-ray results, it has been found that the Cu2SnS3 compound crystallizes in cubic syngony (Lattice par.: a = 0,5438 nm) and the Cu4SnS4 compound crystallizes in the orthorhombic (Lattice par.: a = 1,3487 nm, b = 0,7656 nm, c = 0,6388 nm) structure. The Cu2Sn3S7 compound crystallizes in the monoclinic (Lattice par.: a = 1,2676 nm; b = 0,7346 nm, c = 1,2759 nm, β = 109,600) syngony.
According to DTA results, the Cu4SnS4 combination is melting at 834 °C and the Cu2SnS3 combination is at 855 °C. The temperature more than 673 °C the Cu2Sn3S7 compound is decomposed to Cu2Sn3S7 → Cu2SnS3 + 2SnS2 reaction. Obtained mixture melts at 803 °C. Three endothermic effects were observed at temperatures of 673, 687 and 778 °C in the DTA curved of this compound, which is appropriate for phase transitions of the compound.
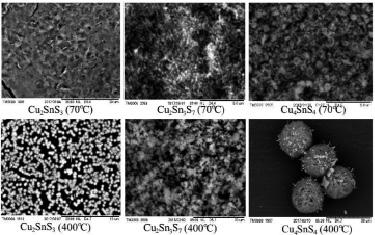
Fig. 3. Microphotos of Cu2SnS3 Cu4SnS4 and Cu2Sn3S7 compounds taken at 70 °C and thermally processed at 400 °C
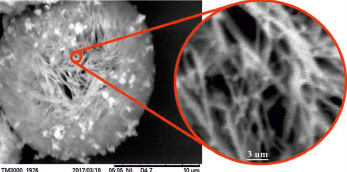
Fig. 4. Microphotos of the surface (10 mkm area) and the internal (3 mkm area) of spherical shaped particles of the Cu4SnS4 compound
Results of elemental analysis of compounds
|
Compound |
The amount of elements, % |
|||||
|
Cu |
Sn |
S |
||||
|
Weight |
atom |
Weight |
atom |
Weight |
atom |
|
|
Cu2SnS3 |
36,69 |
32,83 |
34,79 |
16,67 |
28,52 |
50,50 |
|
Cu4SnS4 |
50,72 |
44,43 |
23,68 |
11,11 |
25,60 |
44,46 |
|
Cu2Sn3S7 |
17,95 |
16,66 |
50,32 |
24,99 |
31,73 |
58,35 |
The Cu2SnS3 Cu4SnS4 and Cu2Sn3S7 compounds micromorphology have been studied by the Hitachi TM3000 brand microscope. Therefore, thin layers of Cu2SnS3 Cu4SnS4 and Cu2Sn3S7 compounds were prepare on glass substrate and SEM photographs were taken (fig. 3). As can be seen from the SEM images, the compounds at 70 °C are essentially amorphous condition. When the thin layers are thermally processing at a vacuum of 400 °C, spherical shape structure formation is observed in Cu2SnS3 and Cu4SnS4 compounds. The Cu2Sn3S7 combination is formed by large aggregates of nanoparticles of non-spherical shaped high adhesion. The Cu2SnS3 compound consists of nanoparticles with a size of 60–100 nm. The Cu4SnS4 compound is spherical shaped nanostructure with a diameter of 15–20 mkm. It has been found that the inside of spheres are composed of nanofilaments (Fig. 4).
As shown in the microphotos, the formation of structure is increasing in this trend Cu2Sn3S7→Cu2SnS3→Cu4SnS4. The reason for this can be explained by the increase of the mass share (18,05 %, 37,32 % and 40,57 %) in this trend. It has been found that, when the T > 500 °C the particles adhere occurs and the relevant structures are broken down. Elemental analysis was carried out (XL Launch Trion dilution refrigerator – OXFORD device) of the contents of obtained sediments to clarify the stoichiometric content of Cu2SnS3, Cu4SnS4 and Cu2Sn3S7 compounds. According to the obtained results, the masses and atomic ratios of copper, tin and sulphide contained was determined in the compounds (Table).
According to the obtained results (table), it has been determined that the prices of experimental determined copper, tin and sulphide mass and atom shares are well compatible with the stoichiometric content of Cu2SnS3, Cu4SnS4 and Cu2Sn3S7 compounds. It was found that, the amount of sulphate in the contents of the compounds is slightly swerve (0,01–0,02 at. %) from the stoichiometry. It is to be explained with this that when adding (CuCl + SnCl4) thioacetamide solution to the initial mixture, certain amount free sulfur is separated because of the condition is acidic (pH = 2–2,5) and which it is remains in the synthesized compounds.
As is known, the properties and structure of the obtained thin layers by the hydrochemical method depend on the number and amount of components of the initial mixture. Therefore, when the synthesis of thiocompounds by the hydrochemical method, determination of the endurance limits of the compounds is considered as one of the main factors. In this respect, the precipitation conditions of the Cu2SnS3 Cu4SnS4 and Cu2Sn3S7 compounds were investigated and it was determined that the maximum yield (96–97 %) of these compounds at 70 °C is observed in the range of pH = 6–8. 0,1 M HCl and 0,1 M NH4OH solutions were used to change the pH of the condition. It has been found that, when the pH > 8 the hydroxothiosalts are obtained in the system and when pH < 2 the relevant compounds are separated.
Conclusion
Cu2SnS3, Cu4SnS4 and Cu2Sn3S7 compounds based on aqueous solutions of CuCl, SnCl4 and CH3CSNH2 compounds were obtained by hydrochemical method and the individuality of the compounds was approved by X-ray and DTA methods. It has been found that, when the obtained sediments in the CuCl–SnCl4–CH3CSNH2–H2O system (pH = 6÷8) at 70 °C perform thermal processing at 400 °C, the nanoparticles of the relevant compounds are formed. When the T>500 °C, nanostructures are broken down because of the particles adhere occurs. When the pH>8, the hydroxothiosalts are obtained in the system.

